The efficacy of neutralizing monoclonal antibodies in transplant recipients with mild-to-moderate COVID-19 |
| |
| Affiliation: | 1. Department of Respiratory Medicine, Tokyo Women''s Medical University, Tokyo, Japan;2. Department of Infectious Diseases, Tokyo Women''s Medical University, Tokyo, Japan;3. Department of Infection Prevention and Control, Tokyo Women''s Medical University, Tokyo, Japan;4. Department of Pharmacy, Tokyo Women''s Medical University, Tokyo, Japan;5. Department of Urology, Tokyo Women''s Medical University, Tokyo, Japan;6. Department of General Medicine, Tokyo Women''s Medical University, Tokyo, Japan;7. Department of Surgery, Institute of Gastroenterology, Tokyo Women''s Medical University, Tokyo, Japan;8. Department of Hematology, Tokyo Women''s Medical University, Tokyo, Japan |
| |
| Abstract: | 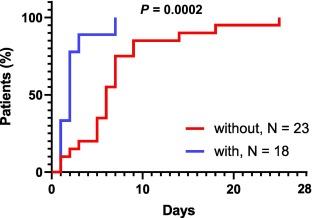
IntroductionTransplant recipients (TRs) are at high risk for severe coronavirus disease 2019 (COVID-19). Neutralizing monoclonal antibodies (mAbs) are used for treating mild-to-moderate COVID-19. However, reports comparing the efficacy of COVID-19 treatment without/with mAbs in TRs are limited. We assessed the efficacy of casirivimab/imdevimab against mild-to-moderate COVID-19 in TRs.MethodsForty-one patients were retrospectively evaluated. The duration until defervescence, oxygen (O2) requirement ≥5 L, and neutralizing antibody levels were compared in TRs with COVID-19 without/with casirivimab/imdevimab.ResultsCasirivimab/imdevimab was correlated with shorter duration until defervescence and non-requirement of O2 ≥ 5 L in TRs with COVID-19 [mean: without/with: 6 vs. 2; P = 0.0002, hazard ratio (HR) = 0.3333, 95% confidence interval (CI) = 0.1763–0.6301; 15 vs. 8; P < 0.0001, HR = 0.5333, 95% CI = 0.2878–0.9883; P = 0.0377, HR = 0.1502, 95% CI = 0.02511–0.8980]. Casirivimab/imdevimab was associated with early defervescence after adjusting for sex and age (P = 0.013, HR = 0.412, 95% CI = 0.205–0.826). The antibody levels between patients without/with casirivimab/imdevimab on the day of hospitalization were not significantly different (P = 0.1055), including 13 TRs with vaccination. Antibody levels were higher in patients with casirivimab/imdevimab at 3–5 days after hospitalization than in those without, at 7–9 days after hospitalization (P < 0.0001, mean, without/with: 414.9/40000 AU/mL).ConclusionCasirivimab/imdevimab was effective and increased the neutralizing antibody in TRs with mild-to-moderate COVID-19, it may contribute toward preventing the progression. |
| |
| Keywords: | |
| 本文献已被 ScienceDirect 等数据库收录! |
|

