Increased dissolution and oral absorption of itraconazole/Soluplus extrudate compared with itraconazole nanosuspension |
| |
| Affiliation: | 1. Institute of Pharmacy, Department of Pharmaceutics, Biopharmaceutics and NutriCosmetics, Freie Universität Berlin, Berlin, Germany;2. Department of Pharmaceutics, Universitas Airlangga, Surabaya, Indonesia;3. Pharmaceutical Development, NextPharma, Berlin, Germany |
| |
| Abstract: | 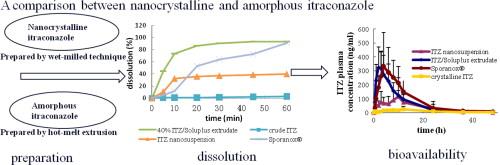
The purpose of this article was to compare the in vitro and in vivo profiles of itraconazole (ITZ) extrudates and nanosuspension separately prepared by two different methods. And it was proved truly to form nanocrystalline and amorphous ITZ characterized by differential scanning calorimetry (DSC), X-ray powder diffraction (XRD) analysis, Fourier transform infrared spectrum (FTIR), transmission electron microscope (TEM), and scanning electron microscope (SEM). The release of ITZ/Soluplus solid dispersions with amorphous ITZ was almost complete while only 40% release was obtained with ITZ nanocrystals. The amorphous state need not to cross over the crystal lattice energy upon dissolution while the crystalline need to overcome it. In the in vivo assay, the AUC(0–t) and Cmax of ITZ/Soluplus were 6.9- and 11.6-time higher than those of pure ITZ. The formulation of the extrudate had an AUC(0–t) and Cmax similar to those of ITZ and also OH-ITZ compared with the commercial capsule (Sporanox®). The relative bioavailability values with their 95% confidence limit were calculated to be 98.3% (92.5–104.1%) and 101.3% (97.9–104.1%), respectively. The results of this study showed increased dissolution and bioavailability of the solid dispersion of Soluplus-based carrier loading ITZ prepared by HME compared with the ITZ nanosuspension prepared by wet milling. |
| |
| Keywords: | Itraconazole Wet milling Nanocrystal Hot melt extrude Amorphous state Dissolution Bioavailability |
| 本文献已被 ScienceDirect 等数据库收录! |
|

