Randomized phase II study to determine the optimal dose of 3-week cycle nab-paclitaxel in patients with metastatic breast cancer |
| |
| Affiliation: | 1. Advanced Cancer Translational Research Institute, Showa University, Tokyo, Japan;2. Department of Breast Medical Oncology, Cancer Institute Hospital of JFCR, Koto, Tokyo, Japan;3. Department of Breast Disease Center, Asahikawa Medical University Hospital, Asahikawa, Japan;4. NHO Hokkaido Cancer Center, Sapporo, Japan;5. Department of Breast Surgery, Kobe City Medical Center General Hospital, Kobe, Japan;6. Teine Keijinkai Hospital, Sapporo, Japan;7. Niigata City General Hospital, Niigata, Japan;8. Department of Breast and Medical Oncology, National Cancer Center Hospital East, Kashiwa, Japan;9. Department of Breast Surgery, Hirosaki Municipal Hospital, Hirosaki, Japan;10. Japanese Red Cross Saitama Hospital, Saitama, Japan;11. Department of Medical Oncology, Kindai University Faculty of Medicine, Osaka-Sayama, Japan;12. Okayama University Hospital, Okayama, Japan;13. Osaka City University Graduate School of Medicine, Osaka, Japan;14. Clinical Research Promotion Center, The University of Tokyo Hospital, Tokyo, Japan;15. Breast Center, Aihara Hospital, Minoh, Japan;p. National Cancer Center Hospital East, Kashiwa, Chiba, Japan |
| |
| Abstract: | 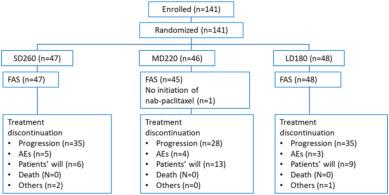
BackgroundChemotherapy-induced peripheral neuropathy is commonly observed in patients treated with nanoparticle albumin–bound paclitaxel (nab-PTX). We conducted a multicenter randomized controlled study to evaluate the optimal dose of nab-PTX.MethodsWe compared three different doses of q3w nab-PTX (Standard: 260 mg/m2 [SD260] vs Medium: 220 mg/m2 [MD220] vs Low: 180 mg/m2 [LD180]) in patients with HER2-negative metastatic breast cancer (MBC). Primary endpoint was progression-free survival (PFS). Grade 3/4 neuropathy rates in the three doses were estimated using the logistic regression model. The optimal dose was selected in two steps. Initially, if the hazard ratio (HR) for PFS was <0.75 or >1.33, the inferior dose was excluded, and we proceeded with the non-inferior dose. Then, if the estimated incidence rate of grade 3/4 neurotoxicity exceeded 10%, that dose was also excluded.ResultsOne hundred forty-one patients were randomly assigned to SD260 (n = 47), MD220 (n = 46), and LD180 (n = 48) groups, and their median PFS was 6.66, 7.34, and 6.82 months, respectively. The HRs were 0.73 (95% confidence interval [CI]: 0.42–1.28) in MD220 vs SD260, 0.77 (95% CI 0.47–1.28) in LD180 vs SD260, and 0.96 (95% CI 0.56–1.66) in LD180 vs MD220. SD260 was inferior to MD220 and was excluded. The estimated incidence rate of grade 3/4 neurotoxicity was 29.5% in SD260, 14.0% in MD220, and 5.9% in LD180. The final selected dose was LD180.ConclusionsIntravenous administration of low-dose nab-PTX at 180 mg/m2 q3w may be the optimal therapy with meaningful efficacy and favorable toxicity in patients with MBC. |
| |
| Keywords: | Nab-paclitaxel Nanoparticle albumin–bound paclitaxel Metastatic breast cancer Solvent-base paclitaxel Chemotherapy-induced peripheral neuropathy CI" },{" #name" :" keyword" ," $" :{" id" :" kwrd0040" }," $$" :[{" #name" :" text" ," _" :" confidence interval CIPN" },{" #name" :" keyword" ," $" :{" id" :" kwrd0050" }," $$" :[{" #name" :" text" ," _" :" chemotherapy-induced peripheral neuropathy CR" },{" #name" :" keyword" ," $" :{" id" :" kwrd0060" }," $$" :[{" #name" :" text" ," _" :" complete remission DCR" },{" #name" :" keyword" ," $" :{" id" :" kwrd0070" }," $$" :[{" #name" :" text" ," _" :" disease control rate DFI" },{" #name" :" keyword" ," $" :{" id" :" kwrd0080" }," $$" :[{" #name" :" text" ," _" :" disease-free interval ECOG" },{" #name" :" keyword" ," $" :{" id" :" kwrd0090" }," $$" :[{" #name" :" text" ," _" :" Eastern Cooperative Oncology Group performance HR" },{" #name" :" keyword" ," $" :{" id" :" kwrd0100" }," $$" :[{" #name" :" text" ," _" :" hazard ratio MBC" },{" #name" :" keyword" ," $" :{" id" :" kwrd0110" }," $$" :[{" #name" :" text" ," _" :" metastatic breast cancer Nab-PTX" },{" #name" :" keyword" ," $" :{" id" :" kwrd0120" }," $$" :[{" #name" :" text" ," _" :" nanoparticle albumin–bound paclitaxel ORR" },{" #name" :" keyword" ," $" :{" id" :" kwrd0130" }," $$" :[{" #name" :" text" ," _" :" overall response rate OS" },{" #name" :" keyword" ," $" :{" id" :" kwrd0140" }," $$" :[{" #name" :" text" ," _" :" overall survival PFS" },{" #name" :" keyword" ," $" :{" id" :" kwrd0150" }," $$" :[{" #name" :" text" ," _" :" progression-free survival PR" },{" #name" :" keyword" ," $" :{" id" :" kwrd0160" }," $$" :[{" #name" :" text" ," _" :" partial response PROs/HRQoL" },{" #name" :" keyword" ," $" :{" id" :" kwrd0170" }," $$" :[{" #name" :" text" ," _" :" patient-reported outcomes/health-related quality-of-life QoL" },{" #name" :" keyword" ," $" :{" id" :" kwrd0180" }," $$" :[{" #name" :" text" ," _" :" quality-of-life RDI" },{" #name" :" keyword" ," $" :{" id" :" kwrd0190" }," $$" :[{" #name" :" text" ," _" :" relative dose intensity RECIST" },{" #name" :" keyword" ," $" :{" id" :" kwrd0200" }," $$" :[{" #name" :" text" ," _" :" response evaluation criteria in solid tumors sb-PTX" },{" #name" :" keyword" ," $" :{" id" :" kwrd0210" }," $$" :[{" #name" :" text" ," _" :" comparing solvent-based paclitaxel TNBC" },{" #name" :" keyword" ," $" :{" id" :" kwrd0220" }," $$" :[{" #name" :" text" ," _" :" triple-negative breast cancer TTF" },{" #name" :" keyword" ," $" :{" id" :" kwrd0230" }," $$" :[{" #name" :" text" ," _" :" time-to-treatment failure |
| 本文献已被 ScienceDirect 等数据库收录! |
|

