Comparison of CTS5 risk model and 21-gene recurrence score assay in large-scale breast cancer population and combination of CTS5 and recurrence score to develop a novel nomogram for prognosis prediction |
| |
| Affiliation: | Department of Breast Surgery, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, 100730, China |
| |
| Abstract: | 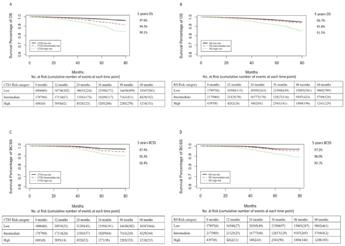
BackgroundBreast cancer is the most common malignancy in women. Clinical models such as Oncotype DX recurrence score (RS) and Clinical Treatment Score post–5 years (CTS5) model for survival prediction are crucial for clinical practice. However, it remains unclear whether CTS5 or RS would be a more powerful clinical model for recurrence risk evaluation. Therefore, we conducted the present study to compare the performance of CTS5 risk model and RS on different recurrence evaluation. And we further integrated the two models into a novel nomogram to improve the power for prognosis prediction.MethodsFemale patients with invasive hormone receptor positive breast cancer in the Surveillance, Epidemiology, and End Results Program (SEER) database with RS data available were included. The clinicopathological data were directly extracted from SEER database. Participants were divided into three subsets according to recurrence timing (<36 months, between 36 and 60 months, and >60 months) for model evaluation. Survival receiver operating characteristic curve and C-index were calculated to evaluate discrimination. Calibration curve were used to visual inspection for calibration. Model comparison was assessed by net reclassification index (NRI) method. Nomogram prognostic model was developed with the combination of CTS5 score and RS and also included other critical clinicopathological parameters.ResultsIn total, 64044 breast cancer patients were enrolled for analysis. The number of patients with survival <36 months (early recurrence subset), 36–60 months (intermediate recurrence subset) and >60 months (late recurrence subset) were 64044, 36878 and 15926, respectively. For model discrimination, CTS5 model was superior to RS for overall survival (OS) prediction (likelihood ratio test P < 0 0.001). RS model showed better performance for breast cancer specific survival (BCSS) in late recurrence subsets and worse performance in early and intermediate recurrence subsets than CTS5 (likelihood ratio test P < 0 0.001). For calibration, CTS5 model was superior to RS model for OS, which overestimated the recurrence risk in low-risk subgroup. Both models overestimated the risk for BCSS. In either early/intermediate/late recurrence patient subsets, there was no significant difference in NRI between two models in terms of both BCSS and OS, indicating the two models had comparable prognostic value. The nomogram which combined these two models largely improved the discrimination and calibration power (C-index 0.70–0.72).ConclusionsOur study proved the CTS5 risk model had comparable prognostic value as RS in HR + breast cancer patients. And the novel nomogram model had better discrimination and calibration than both CTS5 and RS, and future large-scale clinical trials are warranted for further validation. |
| |
| Keywords: | Breast cancer Oncotype Recurrence score Clinical treatment score post–5 years Clinical risk model BC" },{" #name" :" keyword" ," $" :{" id" :" kwrd0040" }," $$" :[{" #name" :" text" ," _" :" Breast cancer CTS5" },{" #name" :" keyword" ," $" :{" id" :" kwrd0050" }," $$" :[{" #name" :" text" ," _" :" Clinical treatment score post–5 years ATAC trial" },{" #name" :" keyword" ," $" :{" id" :" kwrd0060" }," $$" :[{" #name" :" text" ," _" :" Arimidex, Tamoxifen, alone or in combination trial BIG" },{" #name" :" keyword" ," $" :{" id" :" kwrd0070" }," $$" :[{" #name" :" text" ," _" :" Breast International group HR" },{" #name" :" keyword" ," $" :{" id" :" kwrd0080" }," $$" :[{" #name" :" text" ," _" :" Hormone receptor HER2" },{" #name" :" keyword" ," $" :{" id" :" kwrd0090" }," $$" :[{" #name" :" text" ," _" :" Human epidermal growth factor receptor-2 RS" },{" #name" :" keyword" ," $" :{" id" :" kwrd0100" }," $$" :[{" #name" :" text" ," _" :" Recurrence score NSABP" },{" #name" :" keyword" ," $" :{" id" :" kwrd0110" }," $$" :[{" #name" :" text" ," _" :" National surgical adjutant breast project DFS" },{" #name" :" keyword" ," $" :{" id" :" kwrd0120" }," $$" :[{" #name" :" text" ," _" :" Disease-free survival OS" },{" #name" :" keyword" ," $" :{" id" :" kwrd0130" }," $$" :[{" #name" :" text" ," _" :" Overall survival TAILORx" },{" #name" :" keyword" ," $" :{" id" :" kwrd0140" }," $$" :[{" #name" :" text" ," _" :" Trial assigning Individualized options for treatment SEER database" },{" #name" :" keyword" ," $" :{" id" :" kwrd0150" }," $$" :[{" #name" :" text" ," _" :" Surveillance, epidemiology, and end results program BCSS" },{" #name" :" keyword" ," $" :{" id" :" kwrd0160" }," $$" :[{" #name" :" text" ," _" :" Breast cancer specific survival ROC curve" },{" #name" :" keyword" ," $" :{" id" :" kwrd0170" }," $$" :[{" #name" :" text" ," _" :" Survival receiver operating characteristic curve NRI" },{" #name" :" keyword" ," $" :{" id" :" kwrd0180" }," $$" :[{" #name" :" text" ," _" :" Net reclassification index CI" },{" #name" :" keyword" ," $" :{" id" :" kwrd0190" }," $$" :[{" #name" :" text" ," _" :" Confidence interval: Hazard ratio AUC" },{" #name" :" keyword" ," $" :{" id" :" kwrd0200" }," $$" :[{" #name" :" text" ," _" :" Area under curve LN" },{" #name" :" keyword" ," $" :{" id" :" kwrd0210" }," $$" :[{" #name" :" text" ," _" :" lymph node DCA" },{" #name" :" keyword" ," $" :{" id" :" kwrd0220" }," $$" :[{" #name" :" text" ," _" :" Decision curve analysis |
| 本文献已被 ScienceDirect 等数据库收录! |
|

