The effects of lacosamide on cognition,quality-of-life measures,and quality of life in patients with refractory partial epilepsy |
| |
| Affiliation: | 1. Northeast Regional Epilepsy Group, 20 Prospect Ave, Suite 800, Hackensack, NJ 07601, USA;2. Department of Neurological Surgery, Weill Cornell Medicine, 525 E. 68th St., Box 99, New York, NY 10065, USA |
| |
| Abstract: | 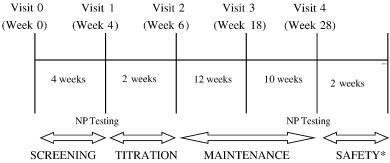
ObjectiveThe objective of this study was to examine cognitive and quality-of-life measures/quality of life outcomes with adjunctive lacosamide therapy in patients with treatment-resistant partial epilepsy.MethodsThis was a prospective, open-label, nonblinded, adjunctive therapy test–retest (within subjects) study of patients with treatment-resistant partial epilepsy in which outcome (cognitive functioning and mood/quality of life) was measured in the same subject before and after adjunctive lacosamide administration for 24 weeks. The cognitive assessment included the following: Controlled Oral Word Association Test, Buschke Selective Reminding Test, Brief Visuospatial Memory Test—Revised, Stroop Color Word Test, Symbol Digit Modalities Test, Digit Span, Digit Cancellation, and Trails A and B. The quality-of-life measures/quality-of-life assessment included the following: Beck Depression Inventory—II, Profile of Mood States, and Quality of Life Inventory—89. Lacosamide was started at 100 mg (50 mg twice daily) and could be titrated as needed up to 400 mg/day (200 mg twice daily). Baseline concomitant AEDs were kept constant. Composite scores were calculated for a pre–post difference score for the cognitive and mood/quality-of-life measures separately and used in regression analyses to correct for the effects of age, education, seizure frequency, seizure severity, dose of lacosamide, and number of AEDs at baseline.ResultsThirty-four patients were enrolled (13 males, 21 females). Mean age was 38.8 ± 2.43 years. Mean seizure frequency decreased significantly from 2.0 ± 2.55 seizures per week at baseline to 1.02 ± 1.72 seizures per week at posttreatment (t = 4.59, p < .0001) with a 50% responder rate seen in 18 patients (52.9%). No significant differences were found on the composite scores of the cognitive or the mood/quality-of-life measures after 6 months of lacosamide.SignificanceLacosamide appeared to have low risks of significant changes in cognition or mood/quality of life. In addition, the present study supports prior studies that have proven lacosamide as an effective adjunctive therapy for the treatment of resistant partial epilepsy. |
| |
| Keywords: | |
| 本文献已被 ScienceDirect 等数据库收录! |
|

