Published online Sep 28, 2022. doi: 10.3748/wjg.v28.i36.5265
Peer-review started: March 12, 2022
First decision: June 11, 2022
Revised: June 20, 2022
Accepted: September 1, 2022
Article in press: September 1, 2022
Published online: September 28, 2022
The intestinal mucosa is a highly compartmentalized structure that forms a direct barrier between the host intestine and the environment, and its dysfunction could result in a serious disease. As T cells, which are important components of the mucosal immune system, interact with gut microbiota and maintain intestinal homeostasis, they may be involved in the process of intestinal barrier dysfunction. P2X7 receptor (P2X7R), a member of the P2X receptors family, mediates the effects of extracellular adenosine triphosphate and is expressed by most innate or adap
Core Tip: Intestinal barrier dysfunction is usually accompanied by inflammation and the death of epithelial cells, which may lead to an elevated concentration of extracellular adenosine triphosphate and the intestinal immune response. Meanwhile, available studies have demonstrated that P2X7 receptor (P2X7R) can be an important regulatory factor in the activation and differentiation of T cells, suggesting that P2X7R may play a key role in intestinal barrier disruption by regulating T cells. Therefore, in this review, we summarized the recent advances regarding the intestinal barrier, the role of P2X7R and T-cells in the pathophysiology of intestinal barrier disruption, and the role of T cell-derived P2X7R in the pathophysiology of intestinal barrier dysfunction.
- Citation: Jiang ZF, Wu W, Hu HB, Li ZY, Zhong M, Zhang L. P2X7 receptor as the regulator of T-cell function in intestinal barrier disruption. World J Gastroenterol 2022; 28(36): 5265-5279
- URL: https://www.wjgnet.com/1007-9327/full/v28/i36/5265.htm
- DOI: https://dx.doi.org/10.3748/wjg.v28.i36.5265
The intestinal tract is one of the largest interfaces in the human body that directly contacts the external environment[1]. The intestine, a highly specialized and complex organ, plays an important role in absorbing useful substances and presenting potentially harmful substances[2]. The intestinal barrier also maintains the homeostasis of the inner environment and develops the intestinal immune system[3]. The intestinal barrier is composed of several parts, including the microbiological barrier, the chemical barrier, the physical barrier and the immune barrier[4]. Dysfunction of the intestinal barrier increases intestinal permeability and is related to the pathophysiology of several serious diseases[5]. The intestinal tract is exposed to various commensal bacteria, dietary antigens and pathogens that are related to immune tolerance and defense, showing the importance of the immune system in the intestine[6]. The immune barrier mainly includes the lamina propria lymphocytes, dendritic cells (DCs), mast cells, macrophages and lymphocytes — mainly CD8+ T cells — located among epithelial cells[7]. Considering the involvement of T cells in the oral tolerance and immune defense against pathogens in the intestine, it is not surprising that they have an essential role in the pathology of intestinal barrier dysfunction[8,9].
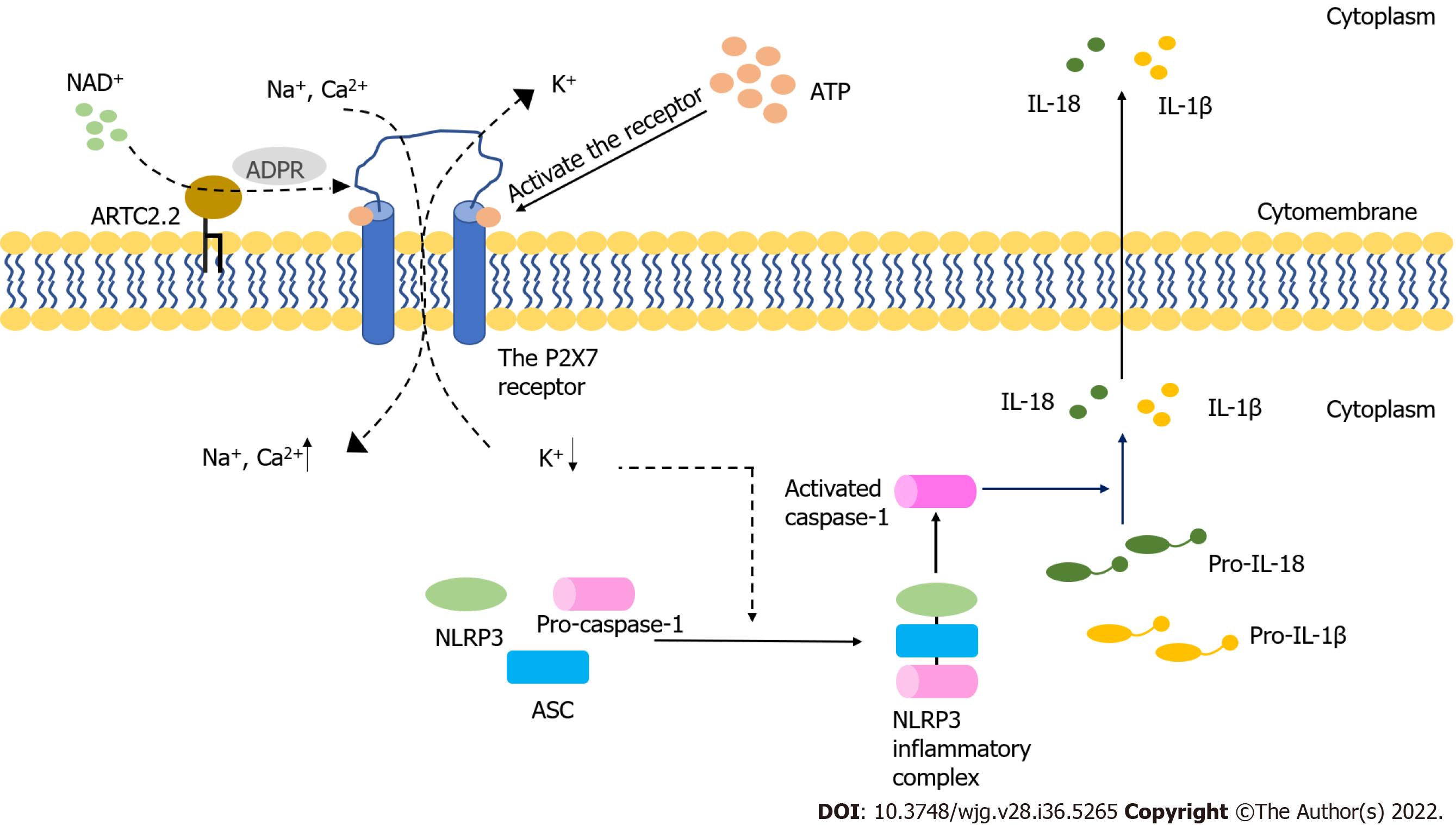
The purinergic signaling pathway is highly conserved and plays a critical role in immune regulatory response[10]. This signaling pathway is mainly mediated by adenosine triphosphate (ATP) and nicotinamide adenine dinucleotide (NAD+). Purinergic receptors are a group of transmembrane proteins widely expressed in immune cells[11]. According to their different structural properties, these receptors can be divided into the following three families: P2X receptor, P2Y receptor and P1 receptor[12]. Among them, P2X receptors, a class of ligand-gated, cationic-selective channels, are mainly activated by extracellular ATP (eATP)[13]. P2X7 receptors (P2X7R) have a low affinity for ATP and need to be triggered by high concentrations of ATP[14]. When the concentration of ATP is low, P2X7R can act as ion channels for Na+, K+ or Ca2+. However, P2X7R can form nonselective and large-conductance pores in settings with high concentrations of ATP, thereby inducing cell apoptosis[14]. Under normal conditions, the cell membrane is impermeable to ATP and other related substances, and the maintenance of low eATP concentration is achieved by the strong degrading activity of ATPases[15,16]. The leakage of intracellular ATP due to the destruction of cell membrane could result in significant elevation of eATP concentration, thereby inducing the activation of the immune system[17-19]. Indeed, an ultrahigh concentration of eATP can be observed at the inflammatory sites[20]. In the process of acute inflammation, ATP activates P2X7R on the Treg cells, inhibiting their activity and viability[21]. P2X receptor channels on effector T cells are stimulated by eATP, facilitating the activation of nuclear factor of activated T cells and the production of IL-2, which could increase the activation of effector T cells[22,23]. The receptor can also be activated by NAD+ released from damaged cells or activated T cells[24,25]. This NAD+-dependent process is associated with ecto-ADP-ribosyltransferase ARTC2.2, which is activated by NAD+ and induces the ADP-ribosylation of P2X7. In the presence of low micromolar concentrations of extracellular NAD+, this process finally leads to cell death because of the activated P2X7R, a phenomenon known as NAD+-induced cell death (NICD)[26,27]. In contrast, NAD+ may be degraded into hydrolysate in the case of high levels of ATP, which would block the NAD+-dependent process[28,29] (Figure 1). Intestinal barrier dysfunction is usually accompanied by inflammation and the death of epithelial cells, which may lead to an elevated concentration of eATP and the intestinal immune response[30]. Meanwhile, available studies have demonstrated that P2X7R can be an important regulatory factor in the activation and differentiation of T cells[31], suggesting that P2X7R may play a key role in intestinal barrier disruption by regulating T cells.
Therefore, in this review, we summarized the recent advances regarding the intestinal barrier, the role of P2X7R and T-cells in the pathophysiology of intestinal barrier disruption, and the role of T cell-derived P2X7R in the pathophysiology of intestinal barrier dysfunction.
The intestinal barrier, one of the most important biological barriers in the body, is composed of various extracellular and cellular components. It works as a semipermeable membrane that allows nutrients to pass through while limiting the transport of pathogens and noxious substances. This dual function is regulated by the interaction among the four components of the intestinal barrier (the microbiological barrier, the chemical barrier, the physical barrier and the immunological barrier)[2].
There are over 1014 microorganisms and about 10000 bacterial species in the human intestine[32]. The microbiological barrier is formed by symbiotic microorganisms in the outermost position of the mucus layer, which effectively prevents harmful substances from entering intestinal epithelial cells[31,33,34]. The chemical barrier, also known as the inner mucus layer, is composed of macromolecules, including proteins, enzymes, peptides and immunoglobulins[35,36]. Mucin2 secreted by goblet cells is the main mucus protein and it serves as a protective barrier[37]. In intestinal crypts, pluripotent stem cells can differentiate into five different cell types, including enterocytes, goblet cells, Paneth cells, enteroendocrine cells and microfold cells[38]. The physical barrier beneath the mucus layer is composed of intestinal epithelial cells which are critical to the physical features of the intestinal barrier[30].
Beneath the intestinal epithelium, the immunological barrier consists of various immune cells, including T lymphocytes, B lymphocytes, dendritic cells, macrophages and plasma cells. This barrier is involved in innate and adaptive immune responses via antigen presentation and the secretion of inflammatory mediators and antibodies[39,40]. In addition to immune cells, substances secreted from these cells are also important in the construction of the intestinal immunological barrier. Secretory IgA, another constituent of the immunological barrier, is mainly found at the intestinal mucosal surface, and it provides antipathogen protection by interacting with bacteria[41].
There are several interactions among different components of the intestinal barrier. The physical barrier and the inner mucus layer separate the microbiological barrier and the intestinal immunological barrier, preventing unnecessary conflict and maintaining intestinal homeostasis[42]. The intestinal microbiota induces the functional maturation of innate and adaptive immunity, and instructs immune response through microbiota-derived metabolites and components (such as lipopolysaccharides and peptidoglycans)[43,44]. The metabolites maintain intestinal homeostasis and regulate inflammation through immune responses, while the components of the microbiota direct immune responses by activating the intestinal TLR pathway[45-47]. For example, the expression of IL17, an inflammatory cytokine produced by γδ T cells, can be inhibited by propionate, a metabolite of intestinal bacteria[48]. Conversely, intestinal immune cells precisely regulate the microbial community both directly and indirectly, thus establishing a sustainable balance between the immune cells and intestinal microbiota[49-51].
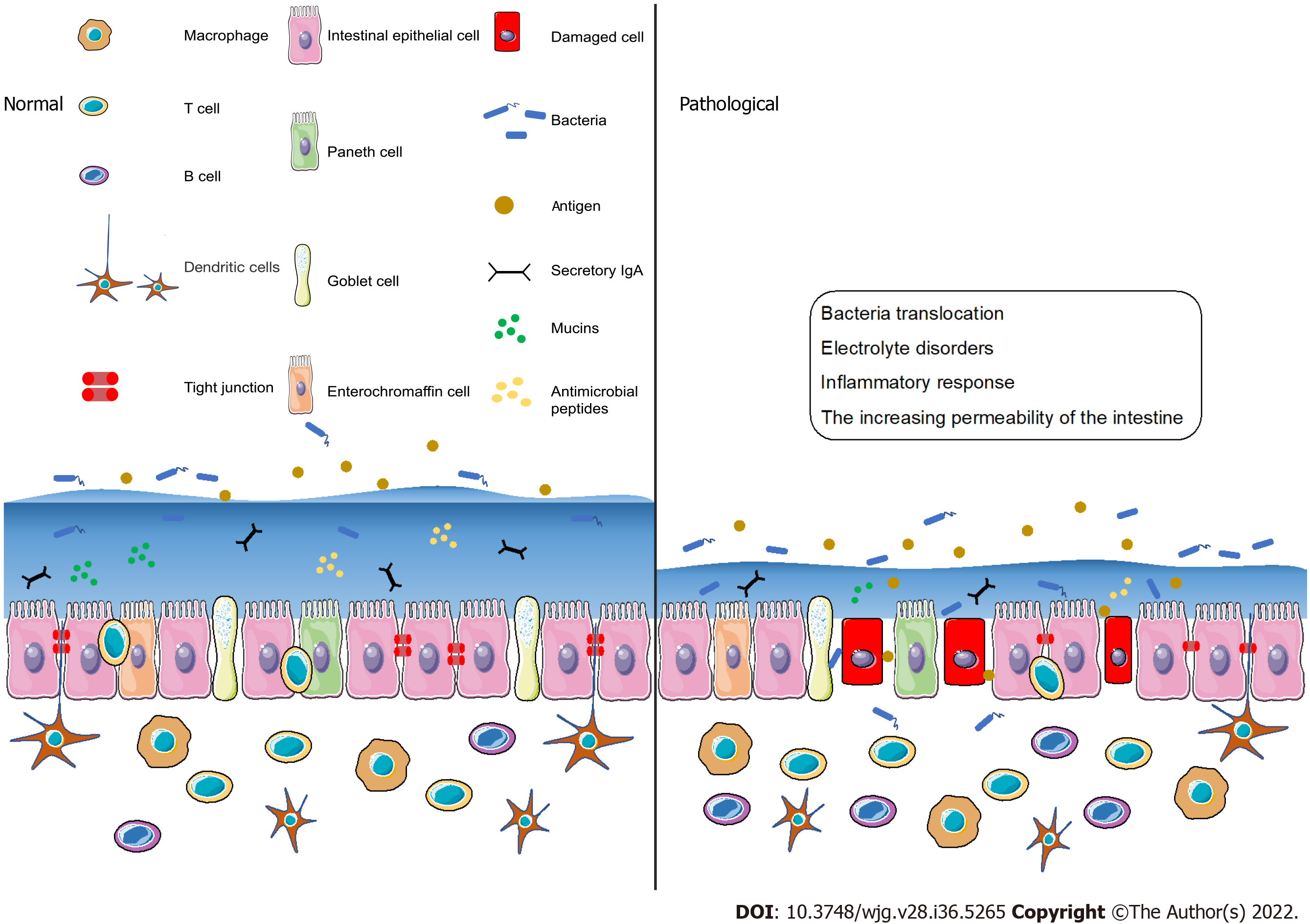
The intestinal barrier should be considered a highly dynamic and complex structure that responds to internal and external stimuli[52,53]. Dysfunction of the intestinal barrier often occurs when the damage of the intestinal mucosa is severe and the components of the intestinal barrier change[54]. Under pathological conditions such as stress[55] and ischemia or hypoxia[56], the intestinal barrier is destroyed and the permeability of the intestine increases, thereby inducing bacterial translocation, electrolyte disorders and inflammatory response[57] (Figure 2). With the increasing permeability of the intestine, locally produced ATP is released into the intestinal microenvironment, followed by the activation of immune cells via ATP receptors, including P2X7 purinoceptor. When the intestinal immune system is activated, the inflammatory effects may not be regulated, which may lead to the irreversible destruction of the intestinal barrier[58]. More recently, it has been reported that increased ATP concentrations promote T-cell responses by enhancing the expression of the CD86 costimulatory molecule on antigen-presenting cells, an effect mediated through P2X7 purinergic receptor. Thus, the immune system may be the key player in barrier dysfunction and T cells may be involved in adaptive immune responses.
T lymphocytes, which are adaptive immune cells, respond to specific antigens and remain the bacterial diversity by complex mechanisms in the homeostatic condition[2]. In the intestine, invariant NKT (iNKT) cells could either enhance or inhibit the immune response, and they might directly or indirectly regulate the microbiota in the intestine[59-62]. CD8+ T cells are the main intraepithelial lymphocytes that monitor and respond to pathogens[63]. CD4+ T cells and T-helper (Th) cells are mainly located in the intestinal lamina propria, and Th1 and Th17 cells can be found in the intestine[64]. Activated intestinal effector T cells could mount immune responses and influence the gut microbiota, and the excess of these cells might induce advanced inflammatory responses and acute or chronic inflammatory diseases[65-67]. In the intestinal adaptive immune response, dendritic cells ingest antigens and activate T cells, and Th cells are induced to differentiate into three different types of Th cells[68]. When lymphocytes respond to different stimuli, they can be divided into different groups based on their cytokine profile, such as Th1, Th2, or Treg cells, which are regulated by P2X7 purinergic receptor, mechanistically[69]. Activated T cells can modulate immune responses by secreting inflammatory cytokines or by interacting with other cells. The function of Th1 cells is to activate and proliferate cytotoxic T cells, thereby inducing the damage of infected intestinal epithelial cells[70]. Th2 cells can release inflammatory cytokines (IL-4, IL-5, and IL-13) and activate B cells to attack the infected cells[71-73]. Transforming growth factor β is capable of suppressing immunoglobulins M and G and promoting their switch to immunoglobulin A. This cytokine is secreted by T cells in Peyer's patches, suggesting the role of T cells in oral tolerance[74]. When intestinal permeability is damaged, antigens can pass through the intestinal epithelial cells and be taken up by macrophages or dendritic cells. Then, the antigens are presented to T cells in the lamina propria by these antigen-presenting cells, which stimulates T cells and induces their proliferation[75,76]. Some antigens may be taken up by intestinal epithelial cells via endocytosis and then be presented to T cells after intracellular processing. This process is based on the classical and nonclassical histocompatibility molecules[77,78]. T cells use both their receptor and a costimulatory signal to recognize antigens[79].
Intestinal barrier disruption is usually accompanied by intestinal inflammation and pathogen invasion. T cells, the key components of adaptive immunity, can effectively limit the invading bacteria and regulate the inflammatory response together with the innate immune system and cytokines[80]. For example, the T helper cell type (Th)1 immune response is necessary in antipathogen protection and is involved in intestinal inflammation[81,82]. In humans, Th17 cells mainly reside in the intestine, where their polarization occurs. Because of the plasticity of Th17 cells, polarized cells have antipathogenic functions and maintain the intestinal epithelial integrity under normal physiological conditions, but they may turn into proinflammatory cells when exposed to IL-23[83]. Th17 cells can mediate inflammation by secreting a proinflammatory cytokine, IL-17A[70]. Peripheral Th17 cells are produced and migrate to the intestine in the case of oral inflammation, which may cause intestinal inflammation[84]. In addition to suppressing the proliferation of Th cells, Treg cells can protect against bacteria and dietary antigens and can produce anti-inflammatory cytokines to exert their anti-inflammatory function, thereby maintaining the homeostasis of the intestinal epithelium[85-87]. With the development of intestinal inflammation, the balance between Th17 cells and Treg cells may be broken up, biasing the function of Th17 cells[88]. In a recent study, it has been found that tissue-resident memory T cells are important in the development of intestinal inflammation, but the role of these cells in this process is not clear[89]. In summary, T cells are very likely to become effective regulatory targets in the intestinal barrier, and T cell-associated therapy may be used in clinical settings in the future.
Among the members of P2X receptor family, P2X7R (encoded by p2rx7) is the largest (with 595 amino acids in humans). It has special structural and signaling features because of its long intracellular carboxy-terminal, which helps prevent receptor desensitization[90,91]. The monomeric structure of P2X7R has two intracellular domains (C-terminal and N-terminal) and an extracellular ATP-binding domain that separates two transmembrane domains[92]. There were over 1500 single nucleotide polymorphisms (SNPs) reported in NCBI database, and most of them were missense, intronic or nonsynonymous[93]. In highly polymorphic human P2RX7, SNPs play a critical role in the biological process and function of P2X7R. About 10 loss of function SNPs and 3 gain of function SNPs have been identified[12]. For example, the activity of human P2X7R was reduced when Ala replaced Glu 496[94]. When Asn replaced lle-568, the expression of P2X7R was decreased to approximately 50% of normal, and P2X7R became nonfunctional[95]. The mutation of R307Q located in the ATP-binding pocket impaired the binding of ATP to P2X7R[96]. Genetic variants in P2X7R may be involved in the inflammatory response[97,98]. P2X7R function related SNPs played a regulatory role in inflammatory diseases[32]. Unlike other P2X receptors, the complete activation of P2X7R requires a higher concentration of ATP (range from about 0.1 to 2.5 mmol/L)[99]. When activated by ATP, P2X7R not only mediates the uptake of cations and macromolecules, but also leads to the activation of intracellular signaling pathways[100-102]. It has been demonstrated that the formation of macropores requires pannexin-1 channels, and pannexin-1 antagonists can decrease the formation of these pores[103]. However, recent data have suggested that the formation of macropores may be intrinsic to P2X7R without accessory molecules[104-106]. Moreover, P2X7R is associated with the activation of the signaling pathway and transcription factors, including MAP kinases, the cyclic AMP response element[107,108]. P2X7R is widely expressed in immune cells, which suggests its importance in the regulation of both the innate and adaptive immunity, especially in the regulation of inflammation[109,110].
ATP is the most important energy molecule and a common extracellular signaling nucleotide that participates in the regulation of cellular proliferation, differentiation and death[111-113]. In a healthy body, eATP is maintained in a low concentration thanks to ATPases in extracellular spaces. ATP can leak from damaged or distressed cells, and can also be released by nonlytic regulated mechanisms, which increase the concentration of eATP16[114-117]. It has been proven that the concentration of eATP is higher in different inflammatory conditions than in normal conditions[118,119].
The intestinal barrier dysfunction induces the inflammatory response and epithelial cell death[120,121]. In the acute-inflammatory tissue, high amounts of IL-6 are released, thereby inducing the synthesis and release of ATP from Treg cells exposed to IL-6[122]. The concentration of eATP may increase after intestinal barrier dysfunction, which may activate P2X7R. T follicular helper cells enhance germinal center reactions by deleting P2X7, resisting ATP-mediated immune cell death[123]. When the concentration of eATP produced by the intestinal microbiota is high, commensal-specific IgA responses initiated by intestinal lymphoid tissues are inhibited, which influences the composition of intestinal microbiota[124,125]. Moreover, Perruzza et al[126] showed that the blockade of P2X7R could decrease proinflammatory cytokines and protect the intestinal barrier function by inhibiting the activation of macrophages. Nucleotide-binding domain, leucine-rich-repeat receptor, pyrin domain-containing NLR family pyrin domain containing 3 (NLRP3) is a multiprotein complex that participates in the occurrence and development of many inflammatory diseases[127]. The inhibition of NLRP3 can reduce intestinal inflammation and enhance the barrier function[128]. Both NLRP3 and P2X7R are expressed in different immune cells, including T cells, B cells and monocytes[99]. Several signaling pathways induced by activated P2X7R may lead to a decrease in intracellular K+, an increase in Ca2+ and the production of reactive oxygen species, which are key steps in NLRP3 activation[129-132] (Figure 1).
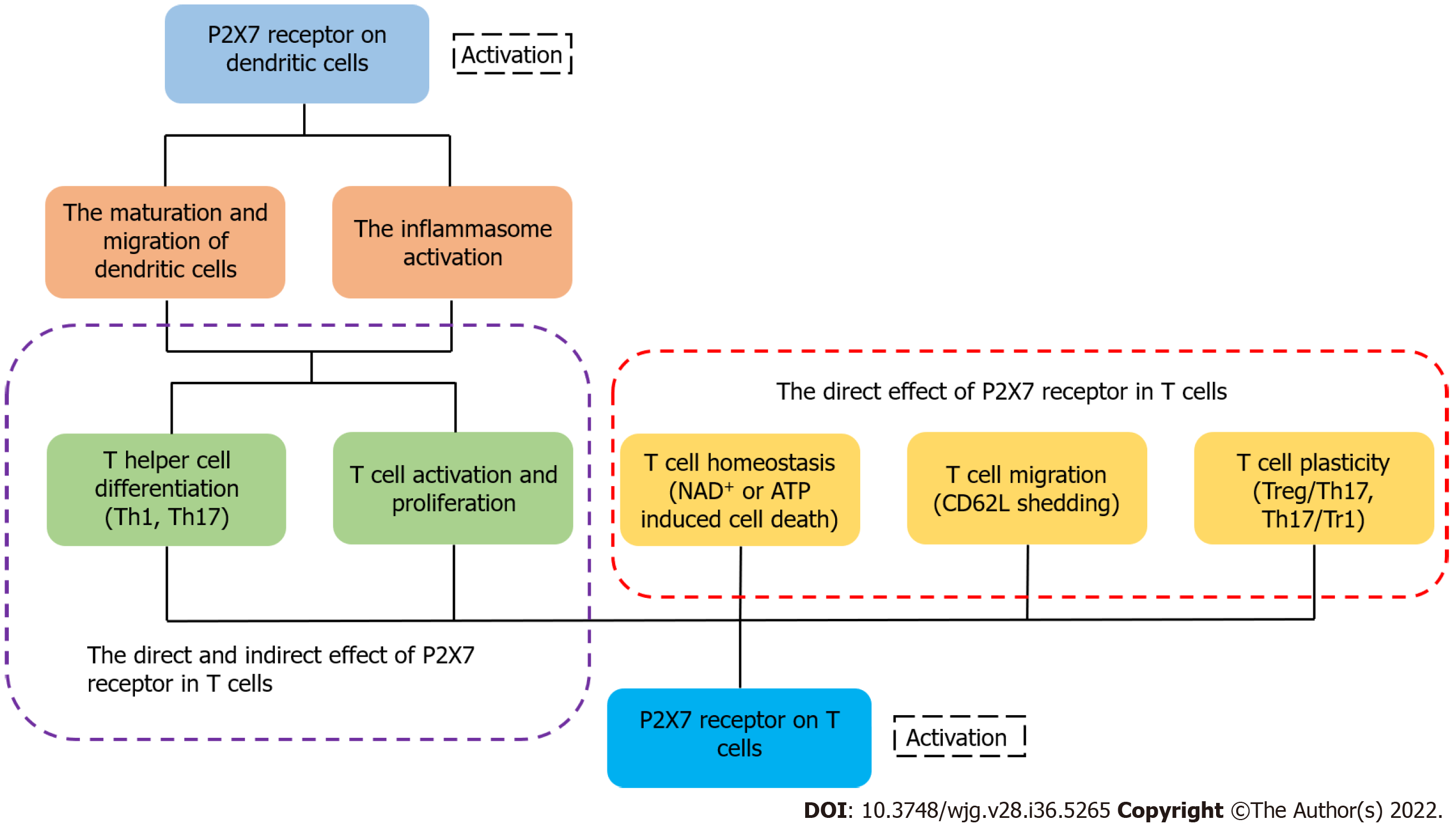
It has been reported that activated P2X7R can affect several of the biological processes of T cells, including activation, differentiation and death[133]. After recognizing antigens, T cells rapidly release ATP through pannexin channels due to the T cell receptor signaling and co-stimulatory molecules[134,135]. Because of the highly expressed P2X7 in iNKT cells, they were susceptible to P2X7-mediated cell death and regulated by vitamin A, finally influencing the intestinal homeostasis[136]. ATP released from T cells can activate P2X receptor which increases the expression of the p2rx7 gene[14,135]. Yip et al[135] found that the silencing of P2X7R blocked Ca2+ influx and inhibited T cell activation in human CD4+ T cells. These findings suggest that activated P2X7R is essential for the activation of T cells. L-selectin (CD62L) is related to the migration of T cells[137,138]. Low expression of L-selectin is necessary for activated or differentiated T cells to egress from the lymph node[139]. P2X7R activated by ATP can trigger CD62L shedding in human naïve T cells[140]. In a lymph node, activated P2X7R also affects the motility of T cells by inducing their calcium waves[141]. When intracellular ATP and NAD+ nucleotides are released from cells, they can trigger the activation of P2X7R and induce apoptosis or necrosis[142]. In the case of low micromolar concentration of extracellular NAD+, ADP ribosylation of P2X7R induces cell death because of persistent P2X7R activation[27]. Under the condition of activated P2X7R, there are two independent ways to induce T cell death: one of them depends on the phosphorylation of ERK1/2, and the other is associated with the nonselective pore[143,144]. In addition, CD62L shedding triggered by the activated P2X7R may induce cell death by apoptosis[145,146]. Compared with native T cells, activated T cells are less sensitive to NICD induced by P2X7R[147]. The expression of P2X7R is different in different populations of T cells. For example, Tregs and follicular helper T cells exhibit high expression of the P2X7 receptor, suggesting that they are more susceptible to cell death than other populations of T cells[148,149]. eATP and P2X7R influence the differentiation of T cells and play a significant role in the metabolism, generation, and memory function of CD8+ T cells[150]. It has been shown that the AMP-activated protein kinase signaling pathway may promote constant efflux of intracellular ATP in memory CD8+ T cells, and is involved in the differentiation and maintenance of memory T cells induced by P2X7R[151,152]. In an inflammatory environment, activated P2X7R drives the differentiation from T cells to Th17 cells[153], and the receptor reduces the differentiation of Tr1 cells with a high expression of IL-10 without Foxp3[21,155]. Activated P2X7R can also regulate the plasticity of Th17 cells and induce Th17 cells to differentiate[156]. In addition to acting directly on T cells, P2X7R can regulate the differentiation of T cells by affecting the physiological functions of dendritic cells[157,158]. Although ATP may not only reduce the DCs-induced Th1 cell differentiation but can also influence the interaction of DCs with T cells, there is little research on the role of P2X7R during this process[159,160]. Moreover, activated P2X7R regulates the cytokine secretion and polarization of Th17 cells by influencing dendritic cells[161,162] (Figure 3). Myeloid derived suppressor cells were considered as the regulator of immunosuppression via affecting the amounts, functions, or phenotypes of T cells, and the ATP/P2X7R signaling axis may be involved in this process[163].
The intestinal barrier dysfunction is a complex and severe pathological condition, which induces the inflammatory response and bacterial invasion. Sepsis is a serious systemic inflammatory disease with high morbidity and mortality in the intensive care unit because it can cause multiple organ failure in patients[164]. Given that the progression and pathogenesis of sepsis have been attributed to intestinal barrier dysfunction, further research on the immune and inflammatory factors of the intestinal barrier dysfunction is necessary[165,166].
According to the above description, T cells are involved in oral tolerance and immune response to antigens in the intestine, and they are the most common lymphocytes that reside in the intestine[167]. Moreover, infiltration by inflammatory T cells is a significant pathological characteristic of intestinal inflammation[168]. Thus, an appropriate number and population of T cells may mitigate the damage of intestinal barrier dysfunction.
P2X7R is widely expressed in T cells and serves as a regulatory factor of their biological processes. Heiss et al[169] found that intestinal CD8+ T cells express a high concentration of P2X7R and are highly sensitive to extracellular nucleotides, indicating that P2X7R can regulate intestinal T cell responses. Inflammatory effector T cells can be depleted and intestinal inflammation can be relieved after treatment with NAD+[170]. P2X7R has been shown to be the trigger for the activation of NLRP3, indicating that this receptor regulates the release of inflammatory cytokines (IL-18, IL-1β) and the initiation of an inflammatory response[171-174]. Therefore, P2X7R may influence inflammation via T cells which is indirect. The selectively P2X7 antagonist was proven to significantly inhibit the innate immune cells and upregulate the immunosuppressive-associated T cells, indicating that this antagonist may be a kind of potential treatment[175]. The effect of P2X7-blockade drug has also been demonstrated in the mouse models with advanced tuberculosis[176]. In addition to the above intracellular signaling pathways (MAPK pathway), previous studies verified that P2X7R also regulated MyD88/NF-κB and PI3K/Akt/mTOR signaling pathways in innate and adaptive immune responses, which suggested that the key proteins in these pathways can be considered as novel therapeutic targets[177].
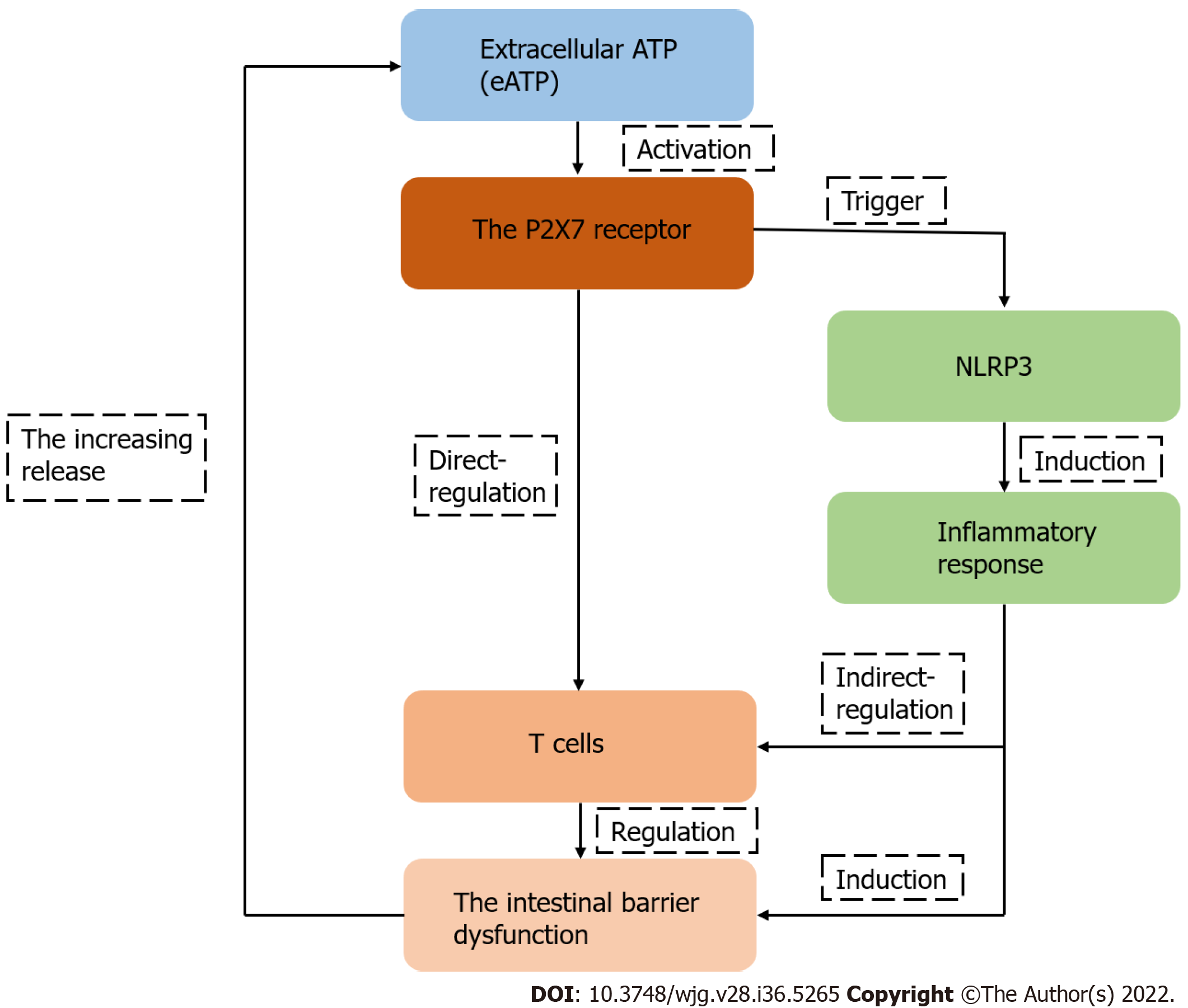
In summary, T cells, the key participant in the intestinal barrier dysfunction, are regulated by P2X7R. The roles and mechanisms of P2X7R are associated with T lymphocytes in the intestinal barrier dysfunction and may be a potential research direction, although there have been few studies on this topic (Figure 4). Furthermore, different specific molecules that inhibit the expression of P2X7R may be potential therapeutic drugs in the future.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Critical care medicine
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B, B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Pongcharoen S, Thailand; Zamani M, Iran S-Editor: Gong ZM L-Editor: Filipodia P-Editor: Gong ZM
| 1. | Takiishi T, Fenero CIM, Câmara NOS. Intestinal barrier and gut microbiota: Shaping our immune responses throughout life. Tissue Barriers. 2017;5:e1373208. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 248] [Cited by in F6Publishing: 441] [Article Influence: 63.0] [Reference Citation Analysis (0)] |
| 2. | Salvo Romero E, Alonso Cotoner C, Pardo Camacho C, Casado Bedmar M, Vicario M. The intestinal barrier function and its involvement in digestive disease. Rev Esp Enferm Dig. 2015;107:686-696. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 66] [Article Influence: 9.4] [Reference Citation Analysis (1)] |
| 3. | Vancamelbeke M, Vermeire S. The intestinal barrier: a fundamental role in health and disease. Expert Rev Gastroenterol Hepatol. 2017;11:821-834. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 489] [Cited by in F6Publishing: 619] [Article Influence: 88.4] [Reference Citation Analysis (0)] |
| 4. | Huang Z, Weng Y, Shen Q, Zhao Y, Jin Y. Microplastic: A potential threat to human and animal health by interfering with the intestinal barrier function and changing the intestinal microenvironment. Sci Total Environ. 2021;785:147365. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 61] [Cited by in F6Publishing: 70] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 5. | Cui Y, Wang Q, Chang R, Zhou X, Xu C. Intestinal Barrier Function-Non-alcoholic Fatty Liver Disease Interactions and Possible Role of Gut Microbiota. J Agric Food Chem. 2019;67:2754-2762. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 95] [Cited by in F6Publishing: 101] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 6. | Izcue A, Coombes JL, Powrie F. Regulatory T cells suppress systemic and mucosal immune activation to control intestinal inflammation. Immunol Rev. 2006;212:256-271. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 357] [Cited by in F6Publishing: 352] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 7. | Gill N, Wlodarska M, Finlay BB. Roadblocks in the gut: barriers to enteric infection. Cell Microbiol. 2011;13:660-669. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 57] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 8. | Olivares-Villagómez D, Van Kaer L. Intestinal Intraepithelial Lymphocytes: Sentinels of the Mucosal Barrier. Trends Immunol. 2018;39:264-275. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 154] [Cited by in F6Publishing: 146] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 9. | Cheroutre H, Lambolez F, Mucida D. The light and dark sides of intestinal intraepithelial lymphocytes. Nat Rev Immunol. 2011;11:445-456. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 419] [Cited by in F6Publishing: 447] [Article Influence: 34.4] [Reference Citation Analysis (0)] |
| 10. | Adinolfi E, Giuliani AL, De Marchi E, Pegoraro A, Orioli E, Di Virgilio F. The P2X7 receptor: A main player in inflammation. Biochem Pharmacol. 2018;151:234-244. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 171] [Cited by in F6Publishing: 249] [Article Influence: 35.6] [Reference Citation Analysis (0)] |
| 11. | Di Virgilio F, Sarti AC, Falzoni S, De Marchi E, Adinolfi E. Extracellular ATP and P2 purinergic signalling in the tumour microenvironment. Nat Rev Cancer. 2018;18:601-618. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 368] [Cited by in F6Publishing: 431] [Article Influence: 71.8] [Reference Citation Analysis (0)] |
| 12. | Di Virgilio F, Dal Ben D, Sarti AC, Giuliani AL, Falzoni S. The P2X7 Receptor in Infection and Inflammation. Immunity. 2017;47:15-31. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 576] [Cited by in F6Publishing: 744] [Article Influence: 106.3] [Reference Citation Analysis (0)] |
| 13. | Niemi K, Teirilä L, Lappalainen J, Rajamäki K, Baumann MH, Öörni K, Wolff H, Kovanen PT, Matikainen S, Eklund KK. Serum amyloid A activates the NLRP3 inflammasome via P2X7 receptor and a cathepsin B-sensitive pathway. J Immunol. 2011;186:6119-6128. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 191] [Cited by in F6Publishing: 213] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 14. | Junger WG. Immune cell regulation by autocrine purinergic signalling. Nat Rev Immunol. 2011;11:201-212. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 559] [Cited by in F6Publishing: 603] [Article Influence: 46.4] [Reference Citation Analysis (0)] |
| 15. | Aliagas E, Muñoz-Esquerre M, Cuevas E, Careta O, Huertas D, López-Sánchez M, Escobar I, Dorca J, Santos S. Is the purinergic pathway involved in the pathology of COPD? Respir Res. 2018;19:103. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 16. | Linden J, Koch-Nolte F, Dahl G. Purine Release, Metabolism, and Signaling in the Inflammatory Response. Annu Rev Immunol. 2019;37:325-347. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 114] [Cited by in F6Publishing: 194] [Article Influence: 38.8] [Reference Citation Analysis (0)] |
| 17. | Burnstock G, Verkhratsky A. Evolutionary origins of the purinergic signalling system. Acta Physiol (Oxf). 2009;195:415-447. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 206] [Cited by in F6Publishing: 211] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 18. | Alarcón-Vila C, Baroja-Mazo A, de Torre-Minguela C, Martínez CM, Martínez-García JJ, Martínez-Banaclocha H, García-Palenciano C, Pelegrin P. CD14 release induced by P2X7 receptor restricts inflammation and increases survival during sepsis. Elife. 2020;9. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 19. | Cekic C, Linden J. Purinergic regulation of the immune system. Nat Rev Immunol. 2016;16:177-192. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 469] [Cited by in F6Publishing: 531] [Article Influence: 66.4] [Reference Citation Analysis (0)] |
| 20. | Pelleg A. Extracellular adenosine 5'-triphosphate in pulmonary disorders. Biochem Pharmacol. 2021;187:114319. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 21. | Schenk U, Frascoli M, Proietti M, Geffers R, Traggiai E, Buer J, Ricordi C, Westendorf AM, Grassi F. ATP inhibits the generation and function of regulatory T cells through the activation of purinergic P2X receptors. Sci Signal. 2011;4:ra12. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 195] [Cited by in F6Publishing: 223] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 22. | Wang L, Jacobsen SE, Bengtsson A, Erlinge D. P2 receptor mRNA expression profiles in human lymphocytes, monocytes and CD34+ stem and progenitor cells. BMC Immunol. 2004;5:16. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 136] [Cited by in F6Publishing: 148] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 23. | Romio M, Reinbeck B, Bongardt S, Hüls S, Burghoff S, Schrader J. Extracellular purine metabolism and signaling of CD73-derived adenosine in murine Treg and Teff cells. Am J Physiol Cell Physiol. 2011;301:C530-C539. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 104] [Cited by in F6Publishing: 118] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 24. | Scheuplein F, Rissiek B, Driver JP, Chen YG, Koch-Nolte F, Serreze DV. A recombinant heavy chain antibody approach blocks ART2 mediated deletion of an iNKT cell population that upon activation inhibits autoimmune diabetes. J Autoimmun. 2010;34:145-154. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 27] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 25. | Bao L, Locovei S, Dahl G. Pannexin membrane channels are mechanosensitive conduits for ATP. FEBS Lett. 2004;572:65-68. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 576] [Cited by in F6Publishing: 612] [Article Influence: 30.6] [Reference Citation Analysis (0)] |
| 26. | Adriouch S, Bannas P, Schwarz N, Fliegert R, Guse AH, Seman M, Haag F, Koch-Nolte F. ADP-ribosylation at R125 gates the P2X7 ion channel by presenting a covalent ligand to its nucleotide binding site. FASEB J. 2008;22:861-869. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 95] [Cited by in F6Publishing: 99] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 27. | Seman M, Adriouch S, Scheuplein F, Krebs C, Freese D, Glowacki G, Deterre P, Haag F, Koch-Nolte F. NAD-induced T cell death: ADP-ribosylation of cell surface proteins by ART2 activates the cytolytic P2X7 purinoceptor. Immunity. 2003;19:571-582. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 245] [Cited by in F6Publishing: 259] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 28. | Lee HC. Structure and enzymatic functions of human CD38. Mol Med. 2006;12:317-323. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 75] [Cited by in F6Publishing: 63] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 29. | Rissiek B, Haag F, Boyer O, Koch-Nolte F, Adriouch S. P2X7 on Mouse T Cells: One Channel, Many Functions. Front Immunol. 2015;6:204. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 73] [Cited by in F6Publishing: 84] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 30. | Jiang ZF, Zhang L. LncRNA: A Potential Research Direction in Intestinal Barrier Function. Dig Dis Sci. 2021;66:1400-1408. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 9] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 31. | Rissiek B, Haag F, Boyer O, Koch-Nolte F, Adriouch S. ADP-ribosylation of P2X7: a matter of life and death for regulatory T cells and natural killer T cells. Curr Top Microbiol Immunol. 2015;384:107-126. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 32] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 32. | Javed I, Cui X, Wang X, Mortimer M, Andrikopoulos N, Li Y, Davis TP, Zhao Y, Ke PC, Chen C. Implications of the Human Gut-Brain and Gut-Cancer Axes for Future Nanomedicine. ACS Nano. 2020;14:14391-14416. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 33. | Paone P, Cani PD. Mucus barrier, mucins and gut microbiota: the expected slimy partners? Gut. 2020;69:2232-2243. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 693] [Cited by in F6Publishing: 632] [Article Influence: 158.0] [Reference Citation Analysis (0)] |
| 34. | Donaldson GP, Lee SM, Mazmanian SK. Gut biogeography of the bacterial microbiota. Nat Rev Microbiol. 2016;14:20-32. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1265] [Cited by in F6Publishing: 1411] [Article Influence: 156.8] [Reference Citation Analysis (0)] |
| 35. | Garrett WS, Gordon JI, Glimcher LH. Homeostasis and inflammation in the intestine. Cell. 2010;140:859-870. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 510] [Cited by in F6Publishing: 531] [Article Influence: 37.9] [Reference Citation Analysis (0)] |
| 36. | Singh PK, Parsek MR, Greenberg EP, Welsh MJ. A component of innate immunity prevents bacterial biofilm development. Nature. 2002;417:552-555. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 753] [Cited by in F6Publishing: 767] [Article Influence: 34.9] [Reference Citation Analysis (0)] |
| 37. | Zarepour M, Bhullar K, Montero M, Ma C, Huang T, Velcich A, Xia L, Vallance BA. The mucin Muc2 limits pathogen burdens and epithelial barrier dysfunction during Salmonella enterica serovar Typhimurium colitis. Infect Immun. 2013;81:3672-3683. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 135] [Cited by in F6Publishing: 145] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 38. | Salim SY, Söderholm JD. Importance of disrupted intestinal barrier in inflammatory bowel diseases. Inflamm Bowel Dis. 2011;17:362-381. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 357] [Cited by in F6Publishing: 402] [Article Influence: 30.9] [Reference Citation Analysis (0)] |
| 39. | Mu Q, Kirby J, Reilly CM, Luo XM. Leaky Gut As a Danger Signal for Autoimmune Diseases. Front Immunol. 2017;8:598. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 266] [Cited by in F6Publishing: 318] [Article Influence: 45.4] [Reference Citation Analysis (0)] |
| 40. | Berin MC, Li H, Sperber K. Antibody-mediated antigen sampling across intestinal epithelial barriers. Ann N Y Acad Sci. 2006;1072:253-261. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 41. | Woof JM, Russell MW. Structure and function relationships in IgA. Mucosal Immunol. 2011;4:590-597. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 211] [Cited by in F6Publishing: 221] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 42. | Kayama H, Okumura R, Takeda K. Interaction Between the Microbiota, Epithelia, and Immune Cells in the Intestine. Annu Rev Immunol. 2020;38:23-48. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 112] [Cited by in F6Publishing: 256] [Article Influence: 85.3] [Reference Citation Analysis (0)] |
| 43. | Round JL, Mazmanian SK. The gut microbiota shapes intestinal immune responses during health and disease. Nat Rev Immunol. 2009;9:313-323. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3154] [Cited by in F6Publishing: 3218] [Article Influence: 214.5] [Reference Citation Analysis (0)] |
| 44. | Rooks MG, Garrett WS. Gut microbiota, metabolites and host immunity. Nat Rev Immunol. 2016;16:341-352. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1428] [Cited by in F6Publishing: 1891] [Article Influence: 270.1] [Reference Citation Analysis (0)] |
| 45. | Park J, Kim M, Kang SG, Jannasch AH, Cooper B, Patterson J, Kim CH. Short-chain fatty acids induce both effector and regulatory T cells by suppression of histone deacetylases and regulation of the mTOR-S6K pathway. Mucosal Immunol. 2015;8:80-93. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 575] [Cited by in F6Publishing: 710] [Article Influence: 78.9] [Reference Citation Analysis (0)] |
| 46. | Sun M, Wu W, Chen L, Yang W, Huang X, Ma C, Chen F, Xiao Y, Zhao Y, Yao S, Carpio VH, Dann SM, Zhao Q, Liu Z, Cong Y. Microbiota-derived short-chain fatty acids promote Th1 cell IL-10 production to maintain intestinal homeostasis. Nat Commun. 2018;9:3555. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 221] [Cited by in F6Publishing: 336] [Article Influence: 56.0] [Reference Citation Analysis (0)] |
| 47. | Diehl GE, Longman RS, Zhang JX, Breart B, Galan C, Cuesta A, Schwab SR, Littman DR. Microbiota restricts trafficking of bacteria to mesenteric lymph nodes by CX(3)CR1(hi) cells. Nature. 2013;494:116-120. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 322] [Cited by in F6Publishing: 343] [Article Influence: 31.2] [Reference Citation Analysis (0)] |
| 48. | Dupraz L, Magniez A, Rolhion N, Richard ML, Da Costa G, Touch S, Mayeur C, Planchais J, Agus A, Danne C, Michaudel C, Spatz M, Trottein F, Langella P, Sokol H, Michel ML. Gut microbiota-derived short-chain fatty acids regulate IL-17 production by mouse and human intestinal γδ T cells. Cell Rep. 2021;36:109332. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 95] [Cited by in F6Publishing: 105] [Article Influence: 35.0] [Reference Citation Analysis (0)] |
| 49. | Levy M, Kolodziejczyk AA, Thaiss CA, Elinav E. Dysbiosis and the immune system. Nat Rev Immunol. 2017;17:219-232. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 724] [Cited by in F6Publishing: 744] [Article Influence: 106.3] [Reference Citation Analysis (0)] |
| 50. | Hoytema van Konijnenburg DP, Reis BS, Pedicord VA, Farache J, Victora GD, Mucida D. Intestinal Epithelial and Intraepithelial T Cell Crosstalk Mediates a Dynamic Response to Infection. Cell. 2017;171:783-794.e13. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 133] [Cited by in F6Publishing: 168] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 51. | Kogut MH, Lee A, Santin E. Microbiome and pathogen interaction with the immune system. Poult Sci. 2020;99:1906-1913. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 82] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 52. | Lee SH. Intestinal permeability regulation by tight junction: implication on inflammatory bowel diseases. Intest Res. 2015;13:11-18. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 408] [Cited by in F6Publishing: 485] [Article Influence: 53.9] [Reference Citation Analysis (0)] |
| 53. | Camilleri M, Madsen K, Spiller R, Greenwood-Van Meerveld B, Verne GN. Intestinal barrier function in health and gastrointestinal disease. Neurogastroenterol Motil. 2012;24:503-512. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 459] [Cited by in F6Publishing: 537] [Article Influence: 44.8] [Reference Citation Analysis (0)] |
| 54. | Nalle SC, Turner JR. Intestinal barrier loss as a critical pathogenic link between inflammatory bowel disease and graft-versus-host disease. Mucosal Immunol. 2015;8:720-730. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 88] [Cited by in F6Publishing: 88] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 55. | Ferrier L, Mazelin L, Cenac N, Desreumaux P, Janin A, Emilie D, Colombel JF, Garcia-Villar R, Fioramonti J, Bueno L. Stress-induced disruption of colonic epithelial barrier: role of interferon-gamma and myosin light chain kinase in mice. Gastroenterology. 2003;125:795-804. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 140] [Cited by in F6Publishing: 159] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 56. | Drewe J, Beglinger C, Fricker G. Effect of ischemia on intestinal permeability of lipopolysaccharides. Eur J Clin Invest. 2001;31:138-144. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 52] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 57. | Pan P, Song Y, Du X, Bai L, Hua X, Xiao Y, Yu X. Intestinal barrier dysfunction following traumatic brain injury. Neurol Sci. 2019;40:1105-1110. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 32] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 58. | Plichta DR, Graham DB, Subramanian S, Xavier RJ. Therapeutic Opportunities in Inflammatory Bowel Disease: Mechanistic Dissection of Host-Microbiome Relationships. Cell. 2019;178:1041-1056. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 113] [Cited by in F6Publishing: 139] [Article Influence: 34.8] [Reference Citation Analysis (0)] |
| 59. | Wingender G, Kronenberg M. Role of NKT cells in the digestive system. IV. The role of canonical natural killer T cells in mucosal immunity and inflammation. Am J Physiol Gastrointest Liver Physiol. 2008;294:G1-G8. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 50] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 60. | Zeissig S, Kaser A, Dougan SK, Nieuwenhuis EE, Blumberg RS. Role of NKT cells in the digestive system. III. Role of NKT cells in intestinal immunity. Am J Physiol Gastrointest Liver Physiol. 2007;293:G1101-G1105. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 32] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 61. | Wang Y, Sedimbi S, Löfbom L, Singh AK, Porcelli SA, Cardell SL. Unique invariant natural killer T cells promote intestinal polyps by suppressing TH1 immunity and promoting regulatory T cells. Mucosal Immunol. 2018;11:131-143. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 48] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 62. | Selvanantham T, Lin Q, Guo CX, Surendra A, Fieve S, Escalante NK, Guttman DS, Streutker CJ, Robertson SJ, Philpott DJ, Mallevaey T. NKT Cell-Deficient Mice Harbor an Altered Microbiota That Fuels Intestinal Inflammation during Chemically Induced Colitis. J Immunol. 2016;197:4464-4472. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 66] [Cited by in F6Publishing: 68] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 63. | Alonso C, Vicario M, Pigrau M, Lobo B, Santos J. Intestinal barrier function and the brain-gut axis. Adv Exp Med Biol. 2014;817:73-113. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 39] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 64. | Maynard CL, Weaver CT. Intestinal effector T cells in health and disease. Immunity. 2009;31:389-400. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 135] [Cited by in F6Publishing: 137] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 65. | Marrack P, Scott-Browne J, MacLeod MK. Terminating the immune response. Immunol Rev. 2010;236:5-10. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 32] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 66. | Yang Y, Torchinsky MB, Gobert M, Xiong H, Xu M, Linehan JL, Alonzo F, Ng C, Chen A, Lin X, Sczesnak A, Liao JJ, Torres VJ, Jenkins MK, Lafaille JJ, Littman DR. Focused specificity of intestinal TH17 cells towards commensal bacterial antigens. Nature. 2014;510:152-156. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 358] [Cited by in F6Publishing: 376] [Article Influence: 37.6] [Reference Citation Analysis (0)] |
| 67. | McKinstry KK, Strutt TM, Swain SL. Regulation of CD4+ T-cell contraction during pathogen challenge. Immunol Rev. 2010;236:110-124. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 52] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 68. | Chen Y, Cui W, Li X, Yang H. Interaction Between Commensal Bacteria, Immune Response and the Intestinal Barrier in Inflammatory Bowel Disease. Front Immunol. 2021;12:761981. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 62] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 69. | Khor B, Gardet A, Xavier RJ. Genetics and pathogenesis of inflammatory bowel disease. Nature. 2011;474:307-317. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1693] [Cited by in F6Publishing: 1721] [Article Influence: 132.4] [Reference Citation Analysis (1)] |
| 70. | Lee SH, Kwon JE, Cho ML. Immunological pathogenesis of inflammatory bowel disease. Intest Res. 2018;16:26-42. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 315] [Cited by in F6Publishing: 306] [Article Influence: 51.0] [Reference Citation Analysis (0)] |
| 71. | Chang JT. Pathophysiology of Inflammatory Bowel Diseases. N Engl J Med. 2020;383:2652-2664. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 274] [Cited by in F6Publishing: 495] [Article Influence: 123.8] [Reference Citation Analysis (0)] |
| 72. | Armstrong H, Alipour M, Valcheva R, Bording-Jorgensen M, Jovel J, Zaidi D, Shah P, Lou Y, Ebeling C, Mason AL, Lafleur D, Jerasi J, Wong GK, Madsen K, Carroll MW, Huynh HQ, Dieleman LA, Wine E. Host immunoglobulin G selectively identifies pathobionts in pediatric inflammatory bowel diseases. Microbiome. 2019;7:1. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 81] [Cited by in F6Publishing: 136] [Article Influence: 27.2] [Reference Citation Analysis (0)] |
| 73. | Romagnani S. Lymphokine production by human T cells in disease states. Annu Rev Immunol. 1994;12:227-257. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 903] [Cited by in F6Publishing: 897] [Article Influence: 29.9] [Reference Citation Analysis (0)] |
| 74. | Baumgart DC, Dignass AU. Intestinal barrier function. Curr Opin Clin Nutr Metab Care. 2002;5:685-694. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 168] [Cited by in F6Publishing: 175] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 75. | Elson CO, Cong Y, Iqbal N, Weaver CT. Immuno-bacterial homeostasis in the gut: new insights into an old enigma. Semin Immunol. 2001;13:187-194. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 34] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 76. | Rescigno M, Urbano M, Valzasina B, Francolini M, Rotta G, Bonasio R, Granucci F, Kraehenbuhl JP, Ricciardi-Castagnoli P. Dendritic cells express tight junction proteins and penetrate gut epithelial monolayers to sample bacteria. Nat Immunol. 2001;2:361-367. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1870] [Cited by in F6Publishing: 1777] [Article Influence: 77.3] [Reference Citation Analysis (0)] |
| 77. | Shao L, Serrano D, Mayer L. The role of epithelial cells in immune regulation in the gut. Semin Immunol. 2001;13:163-176. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 65] [Cited by in F6Publishing: 69] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 78. | Telega GW, Baumgart DC, Carding SR. Uptake and presentation of antigen to T cells by primary colonic epithelial cells in normal and diseased states. Gastroenterology. 2000;119:1548-1559. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 32] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 79. | Cario E, Podolsky DK. Differential alteration in intestinal epithelial cell expression of toll-like receptor 3 (TLR3) and TLR4 in inflammatory bowel disease. Infect Immun. 2000;68:7010-7017. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 942] [Cited by in F6Publishing: 917] [Article Influence: 38.2] [Reference Citation Analysis (0)] |
| 80. | Holleran G, Lopetuso L, Petito V, Graziani C, Ianiro G, McNamara D, Gasbarrini A, Scaldaferri F. The Innate and Adaptive Immune System as Targets for Biologic Therapies in Inflammatory Bowel Disease. Int J Mol Sci. 2017;18. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 46] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 81. | Bagheri N, Salimzadeh L, Shirzad H. The role of T helper 1-cell response in Helicobacter pylori-infection. Microb Pathog. 2018;123:1-8. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 50] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 82. | De Carli M, D'Elios MM, Zancuoghi G, Romagnani S, Del Prete G. Human Th1 and Th2 cells: functional properties, regulation of development and role in autoimmunity. Autoimmunity. 1994;18:301-308. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 65] [Cited by in F6Publishing: 67] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 83. | Shao L, Li M, Zhang B, Chang P. Bacterial dysbiosis incites Th17 cell revolt in irradiated gut. Biomed Pharmacother. 2020;131:110674. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 84. | Kitamoto S, Nagao-Kitamoto H, Jiao Y, Gillilland MG 3rd, Hayashi A, Imai J, Sugihara K, Miyoshi M, Brazil JC, Kuffa P, Hill BD, Rizvi SM, Wen F, Bishu S, Inohara N, Eaton KA, Nusrat A, Lei YL, Giannobile WV, Kamada N. The Intermucosal Connection between the Mouth and Gut in Commensal Pathobiont-Driven Colitis. Cell. 2020;182:447-462.e14. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 144] [Cited by in F6Publishing: 295] [Article Influence: 73.8] [Reference Citation Analysis (0)] |
| 85. | O'Garra A, Vieira P. Regulatory T cells and mechanisms of immune system control. Nat Med. 2004;10:801-805. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 604] [Cited by in F6Publishing: 575] [Article Influence: 28.8] [Reference Citation Analysis (0)] |
| 86. | Fernandes C, Wanderley CWS, Silva CMS, Muniz HA, Teixeira MA, Souza NRP, Cândido AGF, Falcão RB, Souza MHLP, Almeida PRC, Câmara LMC, Lima-Júnior RCP. Role of regulatory T cells in irinotecan-induced intestinal mucositis. Eur J Pharm Sci. 2018;115:158-166. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 87. | Geremia A, Biancheri P, Allan P, Corazza GR, Di Sabatino A. Innate and adaptive immunity in inflammatory bowel disease. Autoimmun Rev. 2014;13:3-10. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 484] [Cited by in F6Publishing: 609] [Article Influence: 55.4] [Reference Citation Analysis (0)] |
| 88. | Ueno A, Jeffery L, Kobayashi T, Hibi T, Ghosh S, Jijon H. Th17 plasticity and its relevance to inflammatory bowel disease. J Autoimmun. 2018;87:38-49. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 141] [Cited by in F6Publishing: 135] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 89. | Zundler S, Becker E, Spocinska M, Slawik M, Parga-Vidal L, Stark R, Wiendl M, Atreya R, Rath T, Leppkes M, Hildner K, López-Posadas R, Lukassen S, Ekici AB, Neufert C, Atreya I, van Gisbergen KPJM, Neurath MF. Hobit- and Blimp-1-driven CD4+ tissue-resident memory T cells control chronic intestinal inflammation. Nat Immunol. 2019;20:288-300. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 107] [Cited by in F6Publishing: 134] [Article Influence: 26.8] [Reference Citation Analysis (0)] |
| 90. | Rassendren F, Buell GN, Virginio C, Collo G, North RA, Surprenant A. The permeabilizing ATP receptor, P2X7. Cloning and expression of a human cDNA. J Biol Chem. 1997;272:5482-5486. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 383] [Cited by in F6Publishing: 400] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 91. | McCarthy AE, Yoshioka C, Mansoor SE. Full-Length P2X7 Structures Reveal How Palmitoylation Prevents Channel Desensitization. Cell. 2019;179:659-670.e13. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 93] [Cited by in F6Publishing: 144] [Article Influence: 28.8] [Reference Citation Analysis (0)] |
| 92. | Surprenant A, Rassendren F, Kawashima E, North RA, Buell G. The cytolytic P2Z receptor for extracellular ATP identified as a P2X receptor (P2X7). Science. 1996;272:735-738. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1356] [Cited by in F6Publishing: 1422] [Article Influence: 50.8] [Reference Citation Analysis (0)] |
| 93. | Zhu X, Li Q, Song W, Peng X, Zhao R. P2X7 receptor: a critical regulator and potential target for breast cancer. J Mol Med (Berl). 2021;99:349-358. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 22] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 94. | Gu BJ, Zhang W, Worthington RA, Sluyter R, Dao-Ung P, Petrou S, Barden JA, Wiley JS. A Glu-496 to Ala polymorphism leads to loss of function of the human P2X7 receptor. J Biol Chem. 2001;276:11135-11142. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 243] [Cited by in F6Publishing: 249] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 95. | Wiley JS, Dao-Ung LP, Li C, Shemon AN, Gu BJ, Smart ML, Fuller SJ, Barden JA, Petrou S, Sluyter R. An Ile-568 to Asn polymorphism prevents normal trafficking and function of the human P2X7 receptor. J Biol Chem. 2003;278:17108-17113. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 129] [Cited by in F6Publishing: 134] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 96. | Gu BJ, Sluyter R, Skarratt KK, Shemon AN, Dao-Ung LP, Fuller SJ, Barden JA, Clarke AL, Petrou S, Wiley JS. An Arg307 to Gln polymorphism within the ATP-binding site causes loss of function of the human P2X7 receptor. J Biol Chem. 2004;279:31287-31295. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 102] [Cited by in F6Publishing: 108] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 97. | Wesselius A, Bours MJ, Arts IC, Theunisz EH, Geusens P, Dagnelie PC. The P2X(7) loss-of-function Glu496Ala polymorphism affects ex vivo cytokine release and protects against the cytotoxic effects of high ATP-levels. BMC Immunol. 2012;13:64. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 98. | Ide S, Nishizawa D, Fukuda K, Kasai S, Hasegawa J, Hayashida M, Minami M, Ikeda K. Haplotypes of P2RX7 gene polymorphisms are associated with both cold pain sensitivity and analgesic effect of fentanyl. Mol Pain. 2014;10:75. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 99. | Tao JH, Cheng M, Tang JP, Dai XJ, Zhang Y, Li XP, Liu Q, Wang YL. Single nucleotide polymorphisms associated with P2X7R function regulate the onset of gouty arthritis. PLoS One. 2017;12:e0181685. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 24] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 100. | Pelegrin P. P2X7 receptor and the NLRP3 inflammasome: Partners in crime. Biochem Pharmacol. 2021;187:114385. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 73] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 101. | Garcia-Marcos M, Pérez-Andrés E, Tandel S, Fontanils U, Kumps A, Kabré E, Gómez-Muñoz A, Marino A, Dehaye JP, Pochet S. Coupling of two pools of P2X7 receptors to distinct intracellular signaling pathways in rat submandibular gland. J Lipid Res. 2006;47:705-714. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 48] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 102. | North RA. P2X receptors. Philos Trans R Soc Lond B Biol Sci. 2016;371. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 106] [Cited by in F6Publishing: 113] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 103. | Virginio C, MacKenzie A, North RA, Surprenant A. Kinetics of cell lysis, dye uptake and permeability changes in cells expressing the rat P2X7 receptor. J Physiol. 1999;519 Pt 2:335-346. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 283] [Cited by in F6Publishing: 295] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 104. | Pelegrin P, Surprenant A. Pannexin-1 mediates large pore formation and interleukin-1beta release by the ATP-gated P2X7 receptor. EMBO J. 2006;25:5071-5082. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1041] [Cited by in F6Publishing: 1135] [Article Influence: 63.1] [Reference Citation Analysis (0)] |
| 105. | Schachter J, Motta AP, de Souza Zamorano A, da Silva-Souza HA, Guimarães MZ, Persechini PM. ATP-induced P2X7-associated uptake of large molecules involves distinct mechanisms for cations and anions in macrophages. J Cell Sci. 2008;121:3261-3270. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 59] [Cited by in F6Publishing: 65] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 106. | Browne LE, Compan V, Bragg L, North RA. P2X7 receptor channels allow direct permeation of nanometer-sized dyes. J Neurosci. 2013;33:3557-3566. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 105] [Cited by in F6Publishing: 118] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 107. | Jiang LH, Rassendren F, Mackenzie A, Zhang YH, Surprenant A, North RA. N-methyl-D-glucamine and propidium dyes utilize different permeation pathways at rat P2X(7) receptors. Am J Physiol Cell Physiol. 2005;289:C1295-C1302. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 101] [Cited by in F6Publishing: 109] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 108. | Amstrup J, Novak I. P2X7 receptor activates extracellular signal-regulated kinases ERK1 and ERK2 independently of Ca2+ influx. Biochem J. 2003;374:51-61. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 76] [Cited by in F6Publishing: 78] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 109. | Gavala ML, Pfeiffer ZA, Bertics PJ. The nucleotide receptor P2RX7 mediates ATP-induced CREB activation in human and murine monocytic cells. J Leukoc Biol. 2008;84:1159-1171. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 25] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 110. | Di Virgilio F, Sarti AC, Grassi F. Modulation of innate and adaptive immunity by P2X ion channels. Curr Opin Immunol. 2018;52:51-59. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 39] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 111. | Alarcón-Vila C, Pizzuto M, Pelegrín P. Purinergic receptors and the inflammatory response mediated by lipids. Curr Opin Pharmacol. 2019;47:90-96. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 112. | Burnstock G. Purinergic signaling and vascular cell proliferation and death. Arterioscler Thromb Vasc Biol. 2002;22:364-373. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 299] [Cited by in F6Publishing: 291] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 113. | Burnstock G. Physiology and pathophysiology of purinergic neurotransmission. Physiol Rev. 2007;87:659-797. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1153] [Cited by in F6Publishing: 1167] [Article Influence: 68.6] [Reference Citation Analysis (0)] |
| 114. | Surprenant A, North RA. Signaling at purinergic P2X receptors. Annu Rev Physiol. 2009;71:333-359. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 402] [Cited by in F6Publishing: 423] [Article Influence: 28.2] [Reference Citation Analysis (0)] |
| 115. | Sun X, Zhou R, Lei Y, Hu J, Li X. The ligand-gated ion channel P2X7 receptor mediates NLRP3/caspase-1-mediated pyroptosis in cerebral cortical neurons of juvenile rats with sepsis. Brain Res. 2020;1748:147109. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 116. | Bulanova E, Bulfone-Paus S. P2 receptor-mediated signaling in mast cell biology. Purinergic Signal. 2010;6:3-17. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 80] [Cited by in F6Publishing: 75] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 117. | Ruan Z, Orozco IJ, Du J, Lü W. Structures of human pannexin 1 reveal ion pathways and mechanism of gating. Nature. 2020;584:646-651. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 110] [Cited by in F6Publishing: 102] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 118. | Chekeni FB, Elliott MR, Sandilos JK, Walk SF, Kinchen JM, Lazarowski ER, Armstrong AJ, Penuela S, Laird DW, Salvesen GS, Isakson BE, Bayliss DA, Ravichandran KS. Pannexin 1 channels mediate 'find-me' signal release and membrane permeability during apoptosis. Nature. 2010;467:863-867. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 751] [Cited by in F6Publishing: 830] [Article Influence: 59.3] [Reference Citation Analysis (0)] |
| 119. | Barberà-Cremades M, Baroja-Mazo A, Gomez AI, Machado F, Di Virgilio F, Pelegrín P. P2X7 receptor-stimulation causes fever via PGE2 and IL-1β release. FASEB J. 2012;26:2951-2962. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 106] [Cited by in F6Publishing: 112] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 120. | Amores-Iniesta J, Barberà-Cremades M, Martínez CM, Pons JA, Revilla-Nuin B, Martínez-Alarcón L, Di Virgilio F, Parrilla P, Baroja-Mazo A, Pelegrín P. Extracellular ATP Activates the NLRP3 Inflammasome and Is an Early Danger Signal of Skin Allograft Rejection. Cell Rep. 2017;21:3414-3426. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 92] [Cited by in F6Publishing: 114] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 121. | Chen WY, Wang M, Zhang J, Barve SS, McClain CJ, Joshi-Barve S. Acrolein Disrupts Tight Junction Proteins and Causes Endoplasmic Reticulum Stress-Mediated Epithelial Cell Death Leading to Intestinal Barrier Dysfunction and Permeability. Am J Pathol. 2017;187:2686-2697. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 62] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 122. | Sun S, Duan Z, Wang X, Chu C, Yang C, Chen F, Wang D, Wang C, Li Q, Ding W. Neutrophil extracellular traps impair intestinal barrier functions in sepsis by regulating TLR9-mediated endoplasmic reticulum stress pathway. Cell Death Dis. 2021;12:606. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 42] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 123. | Piconese S, Gri G, Tripodo C, Musio S, Gorzanelli A, Frossi B, Pedotti R, Pucillo CE, Colombo MP. Mast cells counteract regulatory T-cell suppression through interleukin-6 and OX40/OX40L axis toward Th17-cell differentiation. Blood. 2009;114:2639-2648. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 144] [Cited by in F6Publishing: 154] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 124. | Proietti M, Cornacchione V, Rezzonico Jost T, Romagnani A, Faliti CE, Perruzza L, Rigoni R, Radaelli E, Caprioli F, Preziuso S, Brannetti B, Thelen M, McCoy KD, Slack E, Traggiai E, Grassi F. ATP-gated ionotropic P2X7 receptor controls follicular T helper cell numbers in Peyer's patches to promote host-microbiota mutualism. Immunity. 2014;41:789-801. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 122] [Cited by in F6Publishing: 112] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 125. | Proietti M, Perruzza L, Scribano D, Pellegrini G, D'Antuono R, Strati F, Raffaelli M, Gonzalez SF, Thelen M, Hardt WD, Slack E, Nicoletti M, Grassi F. ATP released by intestinal bacteria limits the generation of protective IgA against enteropathogens. Nat Commun. 2019;10:250. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 58] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 126. | Perruzza L, Gargari G, Proietti M, Fosso B, D'Erchia AM, Faliti CE, Rezzonico-Jost T, Scribano D, Mauri L, Colombo D, Pellegrini G, Moregola A, Mooser C, Pesole G, Nicoletti M, Norata GD, Geuking MB, McCoy KD, Guglielmetti S, Grassi F. T Follicular Helper Cells Promote a Beneficial Gut Ecosystem for Host Metabolic Homeostasis by Sensing Microbiota-Derived Extracellular ATP. Cell Rep. 2017;18:2566-2575. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 63] [Cited by in F6Publishing: 66] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 127. | Wu X, Ren J, Chen G, Wu L, Song X, Li G, Deng Y, Wang G, Gu G, Li J. Systemic blockade of P2X7 receptor protects against sepsis-induced intestinal barrier disruption. Sci Rep. 2017;7:4364. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 43] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 128. | Jiang H, Gong T, Zhou R. The strategies of targeting the NLRP3 inflammasome to treat inflammatory diseases. Adv Immunol. 2020;145:55-93. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 31] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 129. | Li M, Lv R, Wang C, Ge Q, Du H, Lin S. Tricholoma matsutake-derived peptide WFNNAGP protects against DSS-induced colitis by ameliorating oxidative stress and intestinal barrier dysfunction. Food Funct. 2021;12:11883-11897. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 10] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 130. | Lee GS, Subramanian N, Kim AI, Aksentijevich I, Goldbach-Mansky R, Sacks DB, Germain RN, Kastner DL, Chae JJ. The calcium-sensing receptor regulates the NLRP3 inflammasome through Ca2+ and cAMP. Nature. 2012;492:123-127. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 617] [Cited by in F6Publishing: 719] [Article Influence: 59.9] [Reference Citation Analysis (0)] |
| 131. | Tschopp J, Schroder K. NLRP3 inflammasome activation: The convergence of multiple signalling pathways on ROS production? Nat Rev Immunol. 2010;10:210-215. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1162] [Cited by in F6Publishing: 1317] [Article Influence: 94.1] [Reference Citation Analysis (0)] |
| 132. | Hafner-Bratkovič I, Pelegrín P. Ion homeostasis and ion channels in NLRP3 inflammasome activation and regulation. Curr Opin Immunol. 2018;52:8-17. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 50] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 133. | Di A, Xiong S, Ye Z, Malireddi RKS, Kometani S, Zhong M, Mittal M, Hong Z, Kanneganti TD, Rehman J, Malik AB. The TWIK2 Potassium Efflux Channel in Macrophages Mediates NLRP3 Inflammasome-Induced Inflammation. Immunity. 2018;49:56-65.e4. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 157] [Cited by in F6Publishing: 224] [Article Influence: 37.3] [Reference Citation Analysis (0)] |
| 134. | Woehrle T, Yip L, Elkhal A, Sumi Y, Chen Y, Yao Y, Insel PA, Junger WG. Pannexin-1 hemichannel-mediated ATP release together with P2X1 and P2X4 receptors regulate T-cell activation at the immune synapse. Blood. 2010;116:3475-3484. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 219] [Cited by in F6Publishing: 241] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 135. | Yip L, Woehrle T, Corriden R, Hirsh M, Chen Y, Inoue Y, Ferrari V, Insel PA, Junger WG. Autocrine regulation of T-cell activation by ATP release and P2X7 receptors. FASEB J. 2009;23:1685-1693. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 209] [Cited by in F6Publishing: 228] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 136. | Liu Q, Kim CH. Control of Tissue-Resident Invariant NKT Cells by Vitamin A Metabolites and P2X7-Mediated Cell Death. J Immunol. 2019;203:1189-1197. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 137. | Arbonés ML, Ord DC, Ley K, Ratech H, Maynard-Curry C, Otten G, Capon DJ, Tedder TF. Lymphocyte homing and leukocyte rolling and migration are impaired in L-selectin-deficient mice. Immunity. 1994;1:247-260. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 628] [Cited by in F6Publishing: 640] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 138. | Masopust D, Schenkel JM. The integration of T cell migration, differentiation and function. Nat Rev Immunol. 2013;13:309-320. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 382] [Cited by in F6Publishing: 423] [Article Influence: 38.5] [Reference Citation Analysis (0)] |
| 139. | Mueller SN, Gebhardt T, Carbone FR, Heath WR. Memory T cell subsets, migration patterns, and tissue residence. Annu Rev Immunol. 2013;31:137-161. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 540] [Cited by in F6Publishing: 568] [Article Influence: 47.3] [Reference Citation Analysis (0)] |
| 140. | Foster JG, Carter E, Kilty I, MacKenzie AB, Ward SG. Mitochondrial superoxide generation enhances P2X7R-mediated loss of cell surface CD62L on naive human CD4+ T lymphocytes. J Immunol. 2013;190:1551-1559. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 141. | Grassi F. The P2X7 Receptor as Regulator of T Cell Development and Function. Front Immunol. 2020;11:1179. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 31] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 142. | Wang CM, Ploia C, Anselmi F, Sarukhan A, Viola A. Adenosine triphosphate acts as a paracrine signaling molecule to reduce the motility of T cells. EMBO J. 2014;33:1354-1364. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 47] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 143. | Sluyter R. The P2X7 Receptor. Adv Exp Med Biol. 2017;1051:17-53. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 113] [Cited by in F6Publishing: 147] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 144. | Tsukimoto M, Maehata M, Harada H, Ikari A, Takagi K, Degawa M. P2X7 receptor-dependent cell death is modulated during murine T cell maturation and mediated by dual signaling pathways. J Immunol. 2006;177:2842-2850. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 64] [Cited by in F6Publishing: 65] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 145. | Auger R, Motta I, Benihoud K, Ojcius DM, Kanellopoulos JM. A role for mitogen-activated protein kinase(Erk1/2) activation and non-selective pore formation in P2X7 receptor-mediated thymocyte death. J Biol Chem. 2005;280:28142-28151. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 63] [Cited by in F6Publishing: 63] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 146. | Scheuplein F, Schwarz N, Adriouch S, Krebs C, Bannas P, Rissiek B, Seman M, Haag F, Koch-Nolte F. NAD+ and ATP released from injured cells induce P2X7-dependent shedding of CD62L and externalization of phosphatidylserine by murine T cells. J Immunol. 2009;182:2898-2908. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 96] [Cited by in F6Publishing: 104] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 147. | Le Stunff H, Auger R, Kanellopoulos J, Raymond MN. The Pro-451 to Leu polymorphism within the C-terminal tail of P2X7 receptor impairs cell death but not phospholipase D activation in murine thymocytes. J Biol Chem. 2004;279:16918-16926. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 72] [Cited by in F6Publishing: 76] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 148. | Adriouch S, Hubert S, Pechberty S, Koch-Nolte F, Haag F, Seman M. NAD+ released during inflammation participates in T cell homeostasis by inducing ART2-mediated death of naive T cells in vivo. J Immunol. 2007;179:186-194. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 105] [Cited by in F6Publishing: 119] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 149. | Hubert S, Rissiek B, Klages K, Huehn J, Sparwasser T, Haag F, Koch-Nolte F, Boyer O, Seman M, Adriouch S. Extracellular NAD+ shapes the Foxp3+ regulatory T cell compartment through the ART2-P2X7 pathway. J Exp Med. 2010;207:2561-2568. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 135] [Cited by in F6Publishing: 150] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 150. | Iyer SS, Latner DR, Zilliox MJ, McCausland M, Akondy RS, Penaloza-Macmaster P, Hale JS, Ye L, Mohammed AU, Yamaguchi T, Sakaguchi S, Amara RR, Ahmed R. Identification of novel markers for mouse CD4(+) T follicular helper cells. Eur J Immunol. 2013;43:3219-3232. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 45] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 151. | Borges da Silva H, Beura LK, Wang H, Hanse EA, Gore R, Scott MC, Walsh DA, Block KE, Fonseca R, Yan Y, Hippen KL, Blazar BR, Masopust D, Kelekar A, Vulchanova L, Hogquist KA, Jameson SC. The purinergic receptor P2RX7 directs metabolic fitness of long-lived memory CD8+ T cells. Nature. 2018;559:264-268. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 147] [Cited by in F6Publishing: 170] [Article Influence: 28.3] [Reference Citation Analysis (0)] |
| 152. | Wanhainen KM, Jameson SC, da Silva HB. Self-Regulation of Memory CD8 T Cell Metabolism through Extracellular ATP Signaling. Immunometabolism. 2019;1. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 153. | Herzig S, Shaw RJ. AMPK: guardian of metabolism and mitochondrial homeostasis. Nat Rev Mol Cell Biol. 2018;19:121-135. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1378] [Cited by in F6Publishing: 2057] [Article Influence: 293.9] [Reference Citation Analysis (0)] |
| 154. | Roncarolo MG, Gregori S, Battaglia M, Bacchetta R, Fleischhauer K, Levings MK. Interleukin-10-secreting type 1 regulatory T cells in rodents and humans. Immunol Rev. 2006;212:28-50. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 875] [Cited by in F6Publishing: 872] [Article Influence: 48.4] [Reference Citation Analysis (0)] |
| 155. | Mascanfroni ID, Takenaka MC, Yeste A, Patel B, Wu Y, Kenison JE, Siddiqui S, Basso AS, Otterbein LE, Pardoll DM, Pan F, Priel A, Clish CB, Robson SC, Quintana FJ. Metabolic control of type 1 regulatory T cell differentiation by AHR and HIF1-α. Nat Med. 2015;21:638-646. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 304] [Cited by in F6Publishing: 339] [Article Influence: 37.7] [Reference Citation Analysis (0)] |
| 156. | Fernández D, Flores-Santibáñez F, Neira J, Osorio-Barrios F, Tejón G, Nuñez S, Hidalgo Y, Fuenzalida MJ, Meza D, Ureta G, Lladser A, Pacheco R, Acuña-Castillo C, Guixé V, Quintana FJ, Bono MR, Rosemblatt M, Sauma D. Purinergic Signaling as a Regulator of Th17 Cell Plasticity. PLoS One. 2016;11:e0157889. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 24] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 157. | Del Prete A, Scutera S, Sozzani S, Musso T. Role of osteopontin in dendritic cell shaping of immune responses. Cytokine Growth Factor Rev. 2019;50:19-28. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 158. | de Jong EC, Smits HH, Kapsenberg ML. Dendritic cell-mediated T cell polarization. Springer Semin Immunopathol. 2005;26:289-307. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 241] [Cited by in F6Publishing: 234] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 159. | la Sala A, Ferrari D, Corinti S, Cavani A, Di Virgilio F, Girolomoni G. Extracellular ATP induces a distorted maturation of dendritic cells and inhibits their capacity to initiate Th1 responses. J Immunol. 2001;166:1611-1617. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 175] [Cited by in F6Publishing: 176] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 160. | la Sala A, Sebastiani S, Ferrari D, Di Virgilio F, Idzko M, Norgauer J, Girolomoni G. Dendritic cells exposed to extracellular adenosine triphosphate acquire the migratory properties of mature cells and show a reduced capacity to attract type 1 T lymphocytes. Blood. 2002;99:1715-1722. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 96] [Cited by in F6Publishing: 98] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 161. | Li R, Wang J, Li R, Zhu F, Xu W, Zha G, He G, Cao H, Wang Y, Yang J. ATP/P2X7-NLRP3 axis of dendritic cells participates in the regulation of airway inflammation and hyper-responsiveness in asthma by mediating HMGB1 expression and secretion. Exp Cell Res. 2018;366:1-15. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 34] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 162. | Atarashi K, Nishimura J, Shima T, Umesaki Y, Yamamoto M, Onoue M, Yagita H, Ishii N, Evans R, Honda K, Takeda K. ATP drives lamina propria T(H)17 cell differentiation. Nature. 2008;455:808-812. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 815] [Cited by in F6Publishing: 815] [Article Influence: 50.9] [Reference Citation Analysis (0)] |
| 163. | Principi E, Raffaghello L. The role of the P2X7 receptor in myeloid-derived suppressor cells and immunosuppression. Curr Opin Pharmacol. 2019;47:82-89. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 164. | Ayala JC, Grismaldo A, Aristizabal-Pachon AF, Mikhaylenko EV, Nikolenko VN, Mikhaleva LM, Somasundaram SG, Kirkland CE, Aliev G, Morales L. Mitochondrial Dysfunction in Intensive Care Unit Patients. Curr Pharm Des. 2021;27:3074-3081. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 165. | De Winter BY, De Man JG. Interplay between inflammation, immune system and neuronal pathways: effect on gastrointestinal motility. World J Gastroenterol. 2010;16:5523-5535. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 61] [Cited by in F6Publishing: 50] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 166. | Tian X, Li L, Fu G, Wang J, He Q, Zhang C, Qin B. miR-133a-3p regulates the proliferation and apoptosis of intestinal epithelial cells by modulating the expression of TAGLN2. Exp Ther Med. 2021;22:824. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 8] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 167. | Preza GC, Yang OO, Elliott J, Anton PA, Ochoa MT. T lymphocyte density and distribution in human colorectal mucosa, and inefficiency of current cell isolation protocols. PLoS One. 2015;10:e0122723. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 27] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 168. | Tindemans I, Joosse ME, Samsom JN. Dissecting the Heterogeneity in T-Cell Mediated Inflammation in IBD. Cells. 2020;9. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 71] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 169. | Heiss K, Jänner N, Mähnss B, Schumacher V, Koch-Nolte F, Haag F, Mittrücker HW. High sensitivity of intestinal CD8+ T cells to nucleotides indicates P2X7 as a regulator for intestinal T cell responses. J Immunol. 2008;181:3861-3869. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 39] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 170. | Hashimoto-Hill S, Friesen L, Kim M, Kim CH. Contraction of intestinal effector T cells by retinoic acid-induced purinergic receptor P2X7. Mucosal Immunol. 2017;10:912-923. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 33] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 171. | Shokoples BG, Paradis P, Schiffrin EL. P2X7 Receptors: An Untapped Target for the Management of Cardiovascular Disease. Arterioscler Thromb Vasc Biol. 2021;41:186-199. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 43] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 172. | Zhou J, Zhou Z, Liu X, Yin HY, Tang Y, Cao X. P2X7 Receptor-Mediated Inflammation in Cardiovascular Disease. Front Pharmacol. 2021;12:654425. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 19] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 173. | Sharma D, Kanneganti TD. The cell biology of inflammasomes: Mechanisms of inflammasome activation and regulation. J Cell Biol. 2016;213:617-629. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 396] [Cited by in F6Publishing: 478] [Article Influence: 68.3] [Reference Citation Analysis (0)] |
| 174. | Rivas-Yáñez E, Barrera-Avalos C, Parra-Tello B, Briceño P, Rosemblatt MV, Saavedra-Almarza J, Rosemblatt M, Acuña-Castillo C, Bono MR, Sauma D. P2X7 Receptor at the Crossroads of T Cell Fate. Int J Mol Sci. 2020;21. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 29] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 175. | Raffaghello L, Principi E, Baratto S, Panicucci C, Pintus S, Antonini F, Del Zotto G, Benzi A, Bruzzone S, Scudieri P, Minetti C, Gazzerro E, Bruno C. P2X7 Receptor Antagonist Reduces Fibrosis and Inflammation in a Mouse Model of Alpha-Sarcoglycan Muscular Dystrophy. Pharmaceuticals (Basel). 2022;15. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 4] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 176. | Santiago-Carvalho I, de Almeida-Santos G, Bomfim CCB, de Souza PC, Silva JCSE, de Melo BMS, Amaral EP, Cione MVP, Lasunskaia E, Hirata MH, Alves-Filho JCF, Nakaya HI, Alvarez JM, D'Império Lima MR. P2x7 Receptor Signaling Blockade Reduces Lung Inflammation and Necrosis During Severe Experimental Tuberculosis. Front Cell Infect Microbiol. 2021;11:672472. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 14] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 177. | Savio LEB, de Andrade Mello P, da Silva CG, Coutinho-Silva R. The P2X7 Receptor in Inflammatory Diseases: Angel or Demon? Front Pharmacol. 2018;9:52. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 216] [Cited by in F6Publishing: 276] [Article Influence: 46.0] [Reference Citation Analysis (0)] |